当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimal Geometrical Configuration of Cobalt Cations in Spinel Oxides to Promote Oxygen Evolution Reaction.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-27 , DOI: 10.1002/anie.201914245 Zhijuan Liu 1, 2 , Guangjin Wang 3 , Xiaoyan Zhu 1 , Yanyong Wang 1 , Yuqin Zou 1 , Shuangquan Zang 2 , Shuangyin Wang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-27 , DOI: 10.1002/anie.201914245 Zhijuan Liu 1, 2 , Guangjin Wang 3 , Xiaoyan Zhu 1 , Yanyong Wang 1 , Yuqin Zou 1 , Shuangquan Zang 2 , Shuangyin Wang 1
Affiliation
|
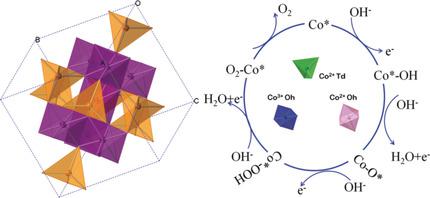
MgCo2 O4 , CoCr2 O4 , and Co2 TiO4 were selected, where only Co3+ in the center of octahedron (Oh), Co2+ in the center of tetrahedron (Td), and Co2+ in the center of Oh, can be active sites for the oxygen evolution reaction (OER). Co3+ (Oh) sites are the best geometrical configuration for OER. Co2+ (Oh) sites exhibit better activity than Co2+ (Td). Calculations demonstrate the conversion of O* into OOH* is the rate-determining step for Co3+ (Oh) and Co2+ (Td). For Co2+ (Oh), it is thermodynamically favorable for the formation of OOH* but difficult for the desorption of O2 . Co3+ (Oh) needs to increase the lowest Gibbs free energy over Co2+ (Oh) and Co2+ (Td), which contributes to the best activity. The coexistence of Co3+ (Oh) and Co2+ (Td) in Co3 O4 can promote the formation of OOH* and decrease the free-energy barrier. This work screens out the optimal geometrical configuration of cobalt cations for OER and gives a valuable principle to design efficient electrocatalysts.
中文翻译:

尖晶石氧化物中钴阳离子的最佳几何构型,以促进氧释放反应。
选择了MgCo2 O4,CoCr2 O4和Co2 TiO4,其中只有八面体(Oh)中心的Co3 +,四面体(Td)中心的Co2 +和Oh中心的Co2 +是氧释放的活性位点反应(OER)。Co3 +(Oh)站点是OER的最佳几何构型。Co2 +(Oh)位点显示出比Co2 +(Td)更好的活性。计算表明,O *转化为OOH *是确定Co3 +(Oh)和Co2 +(Td)的速率的步骤。对于Co2 +(Oh),在热力学上有利于OOH *的形成,但对O2的解吸却很困难。与Co2 +(Oh)和Co2 +(Td)相比,Co3 +(Oh)需要增加最低的吉布斯自由能,这有助于实现最佳活性。Co3 O4中Co3 +(Oh)和Co2 +(Td)的共存可促进OOH *的形成并降低自由能垒。
更新日期:2020-01-31
中文翻译:

尖晶石氧化物中钴阳离子的最佳几何构型,以促进氧释放反应。
选择了MgCo2 O4,CoCr2 O4和Co2 TiO4,其中只有八面体(Oh)中心的Co3 +,四面体(Td)中心的Co2 +和Oh中心的Co2 +是氧释放的活性位点反应(OER)。Co3 +(Oh)站点是OER的最佳几何构型。Co2 +(Oh)位点显示出比Co2 +(Td)更好的活性。计算表明,O *转化为OOH *是确定Co3 +(Oh)和Co2 +(Td)的速率的步骤。对于Co2 +(Oh),在热力学上有利于OOH *的形成,但对O2的解吸却很困难。与Co2 +(Oh)和Co2 +(Td)相比,Co3 +(Oh)需要增加最低的吉布斯自由能,这有助于实现最佳活性。Co3 O4中Co3 +(Oh)和Co2 +(Td)的共存可促进OOH *的形成并降低自由能垒。

































 京公网安备 11010802027423号
京公网安备 11010802027423号