Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2019-12-28 , DOI: 10.1016/j.molliq.2019.112409 Pedro P. Madeira , Mara G. Freire , João A.P. Coutinho
|
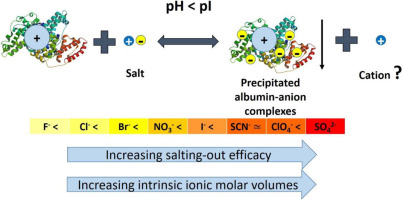
Precipitation experiments of electro-positive albumin by the action of a wide number of salts, and at different concentrations, were performed at a constant temperature (25 °C). The pH range studied covered extreme acidic conditions up to hydronium concentrations where the dissociation of the protein carboxyl groups becomes noticeable. The time required for the clouding phenomenon to occur and the quantity of salted-out protein were also ascertained. The results here reported show that the salt anion is the main salting-out species for the positively charged protein, where their efficacy in salting-out albumin from aqueous solution increases in the order: F− < Cl− < Br− < NO3− < I− < SCN− ~ ClO4− < SO42−. Although at extreme pH conditions the salt cation has no significant influence on the protein salting-out, experiments performed at higher pH values, where the carboxyl groups starts to dissociate, revealed a non-monotonic effect of the salt upon protein precipitation. We interpret this observation as a result of the presence of different protein forms, with which the salt cation participates in chemical equilibrium. Overall, the proteins salting-out phenomenon induced by salt can be rationalized by a general mechanism driven by electrostatic interactions and chemical equilibrium concepts.
中文翻译:

盐阳离子和阴离子在正电白蛋白盐析中的不同作用
在一定温度(25°C)下,通过各种盐的作用和不同的浓度,对正电白蛋白进行沉淀实验。研究的pH范围涵盖了极端酸性条件,直至水合氢盐浓度,其中蛋白质羧基的解离变得很明显。还确定了产生浑浊现象所需的时间和盐析蛋白质的量。结果这里报道表明,该盐的阴离子主要是盐析物种为带正电荷的蛋白,其中它们在盐析白蛋白从水溶液增大的顺序功效:F - <氯- <溴- <NO 3 - <I − <SCN- 〜CLO 4 - <SO 4 2-。尽管在极端pH条件下盐阳离子对蛋白质盐析没有显着影响,但在较高pH值(羧基开始解离)下进行的实验表明,盐对蛋白质沉淀具有非单调作用。我们将此观察结果解释为存在不同蛋白质形式的结果,盐阳离子参与其中的化学平衡。总的来说,由盐引起的蛋白质盐析现象可以通过静电相互作用和化学平衡概念驱动的一般机制来合理化。















































 京公网安备 11010802027423号
京公网安备 11010802027423号