当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unprecedented mode of action of phenothiazines as ionophores unravelled by an NDH-2 bioelectrochemical assay platform
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-27 , DOI: 10.1021/jacs.9b10254 Yoshio Nakatani 1, 2 , Yosuke Shimaki 1 , Debajyoti Dutta 3 , Stephen P Muench 3 , Keith Ireton 1 , Gregory M Cook 1, 2 , Lars J C Jeuken 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-27 , DOI: 10.1021/jacs.9b10254 Yoshio Nakatani 1, 2 , Yosuke Shimaki 1 , Debajyoti Dutta 3 , Stephen P Muench 3 , Keith Ireton 1 , Gregory M Cook 1, 2 , Lars J C Jeuken 3
Affiliation
|
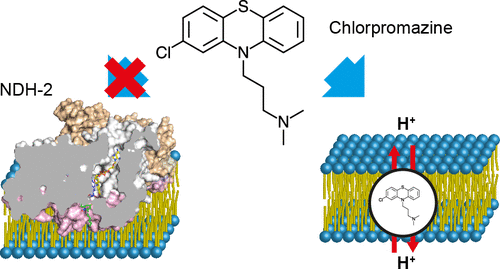
Type II NADH:quinone oxidoreductase (NDH-2) plays a crucial role in the respiratory chains of many organisms. Its absence in mammalian cells makes NDH-2 an attractive new target for developing antimicrobials and anti-protozoal agents. We established a novel bioelectrochemical platform to characterize the catalytic behavior of NDH-2 from Caldalkalibacillus thermarum and Listeria monocytogenes strain EGD-e while bound to native-like lipid membranes. Catalysis of both NADH oxidation and lipophilic quinone reduction by membrane-bound NDH-2 followed the Michaelis-Menten model; however, the maximum turnover was only achieved when a high concentration of quinone (>3 mM) was present in the membrane, suggesting that quinone availability regulates NADH-coupled respiration activity. The quinone analogue 2-heptyl-4-hydroxyquinoline-N-oxide inhibited C. thermarum NDH-2 activity and its potency is higher in a membrane environment compared to assays performed with water-soluble quinone analogues, demonstrating the importance of testing compounds under physiologically relevant conditions. Furthermore, when phenothiazines, one of the most commonly identified NDH-2 inhibitors, were tested, they did not inhibit membrane-bound NDH-2. Instead, our assay platform unexpectedly suggests a novel mode of phenothiazine action where chlorpromazine, a promising anti-tubercular agent and key medicine used to treat psychotic disorders, is able to disrupt pH gradients across bacterial membranes.
中文翻译:

NDH-2生物电化学分析平台揭示吩噻嗪作为离子载体的前所未有的作用模式
II 型 NADH:醌氧化还原酶 (NDH-2) 在许多生物体的呼吸链中起着至关重要的作用。它在哺乳动物细胞中的缺失使 NDH-2 成为开发抗微生物剂和抗原生动物剂的有吸引力的新目标。我们建立了一个新的生物电化学平台来表征来自热碱热碱杆菌和单核细胞增生李斯特菌菌株 EGD-e 的 NDH-2 在与天然类脂质膜结合时的催化行为。膜结合的 NDH-2 对 NADH 氧化和亲脂性醌还原的催化遵循 Michaelis-Menten 模型;然而,只有当膜中存在高浓度的醌(> 3 mM)时才能实现最大转换,这表明醌的可用性调节了 NADH 耦合的呼吸活动。醌类似物 2-heptyl-4-hydroxyquinoline-N-oxide 抑制 C. 与使用水溶性醌类似物进行的测定相比,thermarum NDH-2 活性及其在膜环境中的效力更高,证明了在生理相关条件下测试化合物的重要性。此外,当测试吩噻嗪(最常见的 NDH-2 抑制剂之一)时,它们不会抑制膜结合的 NDH-2。相反,我们的检测平台出人意料地提出了一种吩噻嗪作用的新模式,其中氯丙嗪是一种很有前途的抗结核药物和用于治疗精神病的关键药物,能够破坏细菌膜上的 pH 梯度。证明了在生理相关条件下测试化合物的重要性。此外,当测试吩噻嗪(最常见的 NDH-2 抑制剂之一)时,它们不会抑制膜结合的 NDH-2。相反,我们的检测平台出人意料地提出了一种吩噻嗪作用的新模式,其中氯丙嗪是一种很有前途的抗结核药物和用于治疗精神病的关键药物,能够破坏细菌膜上的 pH 梯度。证明了在生理相关条件下测试化合物的重要性。此外,当测试吩噻嗪(最常见的 NDH-2 抑制剂之一)时,它们不会抑制膜结合的 NDH-2。相反,我们的检测平台出人意料地提出了一种吩噻嗪作用的新模式,其中氯丙嗪是一种很有前途的抗结核药物和用于治疗精神病的关键药物,能够破坏细菌膜上的 pH 梯度。
更新日期:2019-12-27
中文翻译:

NDH-2生物电化学分析平台揭示吩噻嗪作为离子载体的前所未有的作用模式
II 型 NADH:醌氧化还原酶 (NDH-2) 在许多生物体的呼吸链中起着至关重要的作用。它在哺乳动物细胞中的缺失使 NDH-2 成为开发抗微生物剂和抗原生动物剂的有吸引力的新目标。我们建立了一个新的生物电化学平台来表征来自热碱热碱杆菌和单核细胞增生李斯特菌菌株 EGD-e 的 NDH-2 在与天然类脂质膜结合时的催化行为。膜结合的 NDH-2 对 NADH 氧化和亲脂性醌还原的催化遵循 Michaelis-Menten 模型;然而,只有当膜中存在高浓度的醌(> 3 mM)时才能实现最大转换,这表明醌的可用性调节了 NADH 耦合的呼吸活动。醌类似物 2-heptyl-4-hydroxyquinoline-N-oxide 抑制 C. 与使用水溶性醌类似物进行的测定相比,thermarum NDH-2 活性及其在膜环境中的效力更高,证明了在生理相关条件下测试化合物的重要性。此外,当测试吩噻嗪(最常见的 NDH-2 抑制剂之一)时,它们不会抑制膜结合的 NDH-2。相反,我们的检测平台出人意料地提出了一种吩噻嗪作用的新模式,其中氯丙嗪是一种很有前途的抗结核药物和用于治疗精神病的关键药物,能够破坏细菌膜上的 pH 梯度。证明了在生理相关条件下测试化合物的重要性。此外,当测试吩噻嗪(最常见的 NDH-2 抑制剂之一)时,它们不会抑制膜结合的 NDH-2。相反,我们的检测平台出人意料地提出了一种吩噻嗪作用的新模式,其中氯丙嗪是一种很有前途的抗结核药物和用于治疗精神病的关键药物,能够破坏细菌膜上的 pH 梯度。证明了在生理相关条件下测试化合物的重要性。此外,当测试吩噻嗪(最常见的 NDH-2 抑制剂之一)时,它们不会抑制膜结合的 NDH-2。相反,我们的检测平台出人意料地提出了一种吩噻嗪作用的新模式,其中氯丙嗪是一种很有前途的抗结核药物和用于治疗精神病的关键药物,能够破坏细菌膜上的 pH 梯度。

































 京公网安备 11010802027423号
京公网安备 11010802027423号