当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Re‐Engineering Poly(Acrylic Acid) Binder toward Optimized Electrochemical Performance for Silicon Lithium‐Ion Batteries: Branching Architecture Leads to Balanced Properties of Polymeric Binders
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-12-27 , DOI: 10.1002/adfm.201908558 Sisi Jiang 1 , Bin Hu 1 , Zhangxing Shi 1 , Wei Chen 2, 3 , Zhengcheng Zhang 1, 4 , Lu Zhang 1, 4
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-12-27 , DOI: 10.1002/adfm.201908558 Sisi Jiang 1 , Bin Hu 1 , Zhangxing Shi 1 , Wei Chen 2, 3 , Zhengcheng Zhang 1, 4 , Lu Zhang 1, 4
Affiliation
|
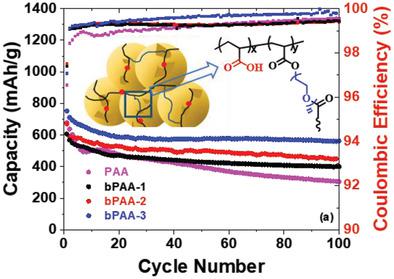
Silicon is a promising anode material for lithium‐ion batteries with its superior capacity. However, the drastic volume changes during lithiation/delithiation cycles hinder the cycling performance, resulting in particle pulverization, conductivity loss, and an unstable electrode–electrolyte interface. Herein, a series of synthetic polymeric binders, poly(acrylic acid‐co‐tetra(ethylene glycol) diacrylate)—featuring a poly(acrylic acid) (PAA) backbone branched via tetra(ethylene glycol) diacrylate (TEGDA)—are developed that edge toward evidencing well‐balanced properties to confront capacity fading in Si‐based electrodes. The incorporation of ether chain not only leads to the branching architecture of the PAA backbone, thus affecting its mechanical properties, but also promotes the conductivity of Li ions. As a result, a synergistic performance improvement is observed in both half and full cells. The best‐performing cell using a branched PAA binder (bPAA) with a feeding molar ratio ([TEGDA]:[acrylic acid(AA)]) of 0.2 results in a 10% increase in initial capacity and a 31% increase in capacity retention over 100 cycles compared to the linear PAA cell. The cross‐sectional microscopic images of the cycled electrodes reveal that bPAA binders can drastically reduce the electrode expansion. This improvement results from the well‐balanced properties of the polymer design, which could guide further development for more advanced binder materials.
中文翻译:

重新设计聚丙烯酸粘合剂,以优化硅锂离子电池的电化学性能:分支结构导致聚合物粘合剂的平衡性能
硅具有出色的容量,是锂离子电池的有希望的负极材料。但是,在锂化/脱锂循环中,体积的急剧变化会阻碍循环性能,从而导致颗粒粉碎,电导率损失以及不稳定的电极-电解质界面。在本文中,一系列合成聚合物粘合剂,聚(丙烯酸-共‐四(乙二醇)二丙烯酸酯)-具有通过四丙烯酸(乙二醇)(TEGDA)分支的聚(丙烯酸)(PAA)主链的特性-开发出了证明具有良好平衡特性的优势,以应对硅基材料的容量衰减电极。醚链的结合不仅导致PAA主链的分支结构,从而影响其机械性能,而且还促进了Li离子的电导率。结果,在半电池和全电池中均观察到协同性能的提高。使用支链PAA粘合剂(bPAA)且进料摩尔比([TEGDA]:[丙烯酸(AA)])为0.2的最佳电池可将初始容量提高10%,将容量保持率提高31%相较于线性PAA电池,其周期超过100个。循环电极的横截面显微图像显示,bPAA粘合剂可以大大减少电极的膨胀。这项改进来自聚合物设计的均衡特性,可以指导更高级粘合剂材料的进一步开发。
更新日期:2020-03-03
中文翻译:

重新设计聚丙烯酸粘合剂,以优化硅锂离子电池的电化学性能:分支结构导致聚合物粘合剂的平衡性能
硅具有出色的容量,是锂离子电池的有希望的负极材料。但是,在锂化/脱锂循环中,体积的急剧变化会阻碍循环性能,从而导致颗粒粉碎,电导率损失以及不稳定的电极-电解质界面。在本文中,一系列合成聚合物粘合剂,聚(丙烯酸-共‐四(乙二醇)二丙烯酸酯)-具有通过四丙烯酸(乙二醇)(TEGDA)分支的聚(丙烯酸)(PAA)主链的特性-开发出了证明具有良好平衡特性的优势,以应对硅基材料的容量衰减电极。醚链的结合不仅导致PAA主链的分支结构,从而影响其机械性能,而且还促进了Li离子的电导率。结果,在半电池和全电池中均观察到协同性能的提高。使用支链PAA粘合剂(bPAA)且进料摩尔比([TEGDA]:[丙烯酸(AA)])为0.2的最佳电池可将初始容量提高10%,将容量保持率提高31%相较于线性PAA电池,其周期超过100个。循环电极的横截面显微图像显示,bPAA粘合剂可以大大减少电极的膨胀。这项改进来自聚合物设计的均衡特性,可以指导更高级粘合剂材料的进一步开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号