当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-Arylpiperidines via Pd-Catalyzed Arylation of Aza-Achmatowicz Rearrangement Products with Arylboronic Acids.
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.orglett.9b04220 Guodong Zhao 1 , Daniel P Canterbury 2 , Alexandria P Taylor 2 , Xiayun Cheng 2 , Peter Mikochik 2 , Scott W Bagley 2 , Rongbiao Tong 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.orglett.9b04220 Guodong Zhao 1 , Daniel P Canterbury 2 , Alexandria P Taylor 2 , Xiayun Cheng 2 , Peter Mikochik 2 , Scott W Bagley 2 , Rongbiao Tong 1
Affiliation
|
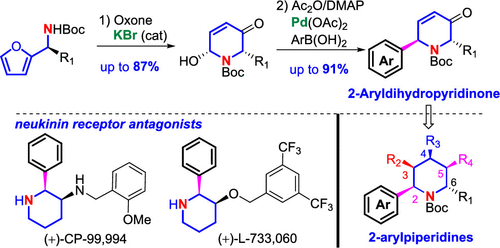
The first Pd-catalyzed arylation of aza-Achmatowicz rearrangement products with arylboronic acids is achieved, providing versatile 2-aryldihydropyridinones for facile synthesis of highly functionalized 2-arylpiperidines. Key to this arylation is the use of non-phosphine-ligand palladium precatalyst. The substrate scope is demonstrated with >26 examples, and the utility of 2-aryldihydropyridinones is illustrated by the synthesis of a small collection of 2-arylpiperidines with substituents or functional groups at any carbon (C2-C6) as well as two NK1 receptor antagonists (+)-CP-999,94 and (+)-L-733,060.
中文翻译:

通过芳族硼酸经Pd催化的Aza-Achmatowicz重排产物的芳基化反应合成2-芳基哌啶。
实现了Aza-Achmatowicz重排产物与芳基硼酸的第一个Pd催化的芳基化反应,从而提供了通用的2-芳基二氢吡啶酮类化合物,可轻松合成高度官能化的2-芳基哌啶。芳基化的关键是使用非膦配体钯预催化剂。用> 26个实例证明了底物的范围,并且通过合成少量的在任何碳原子上具有取代基或官能团的2-芳基哌啶以及两个NK1受体拮抗剂的合成,说明了2-芳基二氢吡啶酮的用途。 (+)-CP-999,94和(+)-L-733,060。
更新日期:2019-12-27
中文翻译:

通过芳族硼酸经Pd催化的Aza-Achmatowicz重排产物的芳基化反应合成2-芳基哌啶。
实现了Aza-Achmatowicz重排产物与芳基硼酸的第一个Pd催化的芳基化反应,从而提供了通用的2-芳基二氢吡啶酮类化合物,可轻松合成高度官能化的2-芳基哌啶。芳基化的关键是使用非膦配体钯预催化剂。用> 26个实例证明了底物的范围,并且通过合成少量的在任何碳原子上具有取代基或官能团的2-芳基哌啶以及两个NK1受体拮抗剂的合成,说明了2-芳基二氢吡啶酮的用途。 (+)-CP-999,94和(+)-L-733,060。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号