当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
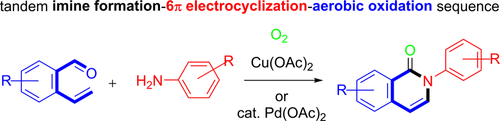
Tandem Reaction Approaches to Isoquinolones from 2-Vinylbenzaldehydes and Anilines via Imine Formation-6π-Electrocyclization-Aerobic Oxidation Sequence.
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.orglett.9b04233 Jiyeon Lee 1 , Hun Young Kim 1 , Kyungsoo Oh 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.orglett.9b04233 Jiyeon Lee 1 , Hun Young Kim 1 , Kyungsoo Oh 1
Affiliation
|
Two distinctive transition-metal-promoted aerobic oxidation protocols have been developed for the synthesis of isoquinolones from 2-vinylbenzaldehydes and aniline derivatives. Thus, the one-pot tandem reaction sequence of imine formation, thermal 6π-electrocyclization, followed by either Cu(OAc)2-mediated or Pd(OAc)2-catalyzed aerobic oxidation protocol allowed the ready access to isoquinolone derivatives. The control experiments revealed that the 1,2-dihydroisoquinoline intermediates from the 6π-electrocyclization of 1-azatrienes were aerobically oxidized to isoquinolones in the presence of either Cu(OAc)2 or Pd(OAc)2 catalyst.
中文翻译:

通过亚胺形成-6π-电环化-好氧氧化序列从2-乙烯基苯甲醛和苯胺生成异喹诺酮的串联反应方法。
已经开发出两种独特的过渡金属促进的需氧氧化方案,用于从2-乙烯基苯甲醛和苯胺衍生物合成异喹诺酮。因此,亚胺形成,热6π-电环化,然后由Cu(OAc)2介导的或Pd(OAc)2催化的好氧氧化协议的一锅串联反应序列使现成的异喹诺酮衍生物易于使用。对照实验表明,在Cu(OAc)2或Pd(OAc)2催化剂存在下,1-氮杂环丁烷的6π-电环化形成的1,2-二氢异喹啉中间体被好氧氧化为异喹诺酮。
更新日期:2019-12-27
中文翻译:

通过亚胺形成-6π-电环化-好氧氧化序列从2-乙烯基苯甲醛和苯胺生成异喹诺酮的串联反应方法。
已经开发出两种独特的过渡金属促进的需氧氧化方案,用于从2-乙烯基苯甲醛和苯胺衍生物合成异喹诺酮。因此,亚胺形成,热6π-电环化,然后由Cu(OAc)2介导的或Pd(OAc)2催化的好氧氧化协议的一锅串联反应序列使现成的异喹诺酮衍生物易于使用。对照实验表明,在Cu(OAc)2或Pd(OAc)2催化剂存在下,1-氮杂环丁烷的6π-电环化形成的1,2-二氢异喹啉中间体被好氧氧化为异喹诺酮。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号