当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of the Oxidation of 3,3',5,5'-Tetramethylbenzidine Catalyzed by Peroxidase-Like Pt Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes: A Kinetic Study.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-02-03 , DOI: 10.1002/cphc.201901087 Sasa Gu 1 , Sebastian Risse 2 , Yan Lu 2, 3 , Matthias Ballauff 2
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-02-03 , DOI: 10.1002/cphc.201901087 Sasa Gu 1 , Sebastian Risse 2 , Yan Lu 2, 3 , Matthias Ballauff 2
Affiliation
|
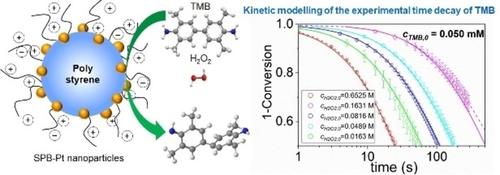
Experimental and kinetic modelling studies are presented to investigate the mechanism of 3,3′,5,5′‐tetramethylbenzidine (TMB) oxidation by hydrogen peroxide (H2O2) catalyzed by peroxidase‐like Pt nanoparticles immobilized in spherical polyelectrolyte brushes (SPB−Pt). Due to the high stability of SPB−Pt colloidal, this reaction can be monitored precisely in situ by UV/VIS spectroscopy. The time‐dependent concentration of the blue‐colored oxidation product of TMB expressed by different kinetic models was used to simulate the experimental data by a genetic fitting algorithm. After falsifying the models with abundant experimental data, it is found that both H2O2 and TMB adsorb on the surface of Pt nanoparticles to react, indicating that the reaction follows the Langmuir–Hinshelwood mechanism. A true rate constant k, characterizing the rate‐determining step of the reaction and which is independent on the amount of catalysts used, is obtained for the first time. Furthermore, it is found that the product adsorbes strongly on the surface of nanoparticles, thus inhibiting the reaction. The entire analysis provides a new perspective to study the catalytic mechanism and evaluate the catalytic activity of the peroxidase‐like nanoparticles.
中文翻译:

固定在球形聚电解质电刷中的过氧化物酶样Pt纳米颗粒催化3,3',5,5'-四甲基联苯胺氧化的机理:动力学研究。
进行了实验和动力学建模研究,以研究固定在球形聚电解质电刷(SPB)中的过氧化物酶样Pt纳米颗粒催化的过氧化氢(H 2 O 2)催化3,3',5,5'-四甲基联苯胺(TMB)氧化的机理-Pt)。由于SPB-Pt胶体的高度稳定性,可以通过UV / VIS光谱法精确地原位监测该反应。不同动力学模型表达的TMB的蓝色氧化产物随时间的浓度通过遗传拟合算法用于模拟实验数据。在通过大量实验数据对模型进行伪造后,发现两种H 2 O 2TMB和TMB吸附在Pt纳米颗粒的表面上发生反应,表明该反应遵循Langmuir-Hinshelwood机理。首次获得表征反应速率确定步骤且与催化剂用量无关的真实速率常数k。此外,发现产物强烈吸附在纳米颗粒的表面上,从而抑制了反应。整个分析为研究过氧化物酶样纳米颗粒的催化机理和评估其催化活性提供了新的视角。
更新日期:2020-02-03
中文翻译:

固定在球形聚电解质电刷中的过氧化物酶样Pt纳米颗粒催化3,3',5,5'-四甲基联苯胺氧化的机理:动力学研究。
进行了实验和动力学建模研究,以研究固定在球形聚电解质电刷(SPB)中的过氧化物酶样Pt纳米颗粒催化的过氧化氢(H 2 O 2)催化3,3',5,5'-四甲基联苯胺(TMB)氧化的机理-Pt)。由于SPB-Pt胶体的高度稳定性,可以通过UV / VIS光谱法精确地原位监测该反应。不同动力学模型表达的TMB的蓝色氧化产物随时间的浓度通过遗传拟合算法用于模拟实验数据。在通过大量实验数据对模型进行伪造后,发现两种H 2 O 2TMB和TMB吸附在Pt纳米颗粒的表面上发生反应,表明该反应遵循Langmuir-Hinshelwood机理。首次获得表征反应速率确定步骤且与催化剂用量无关的真实速率常数k。此外,发现产物强烈吸附在纳米颗粒的表面上,从而抑制了反应。整个分析为研究过氧化物酶样纳米颗粒的催化机理和评估其催化活性提供了新的视角。






























 京公网安备 11010802027423号
京公网安备 11010802027423号