Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced excited-state hydrogen bonding strengthening of hemiindigo for the drastically fluorescence quenching in protic solvent and water sensing in aprotic solvent
Journal of Luminescence ( IF 3.3 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jlumin.2019.116993 Xi Zhao , Songqiu Yang
Journal of Luminescence ( IF 3.3 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jlumin.2019.116993 Xi Zhao , Songqiu Yang
|
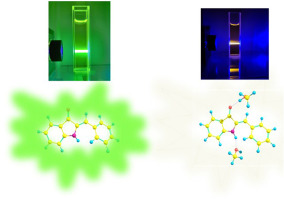
Abstract Hydrogen bonding in excited state could regulate the photochemical and photophysical processes of organic molecules. In this work, the fluorescence and photoisomerization properties of Hemiindigo (HI) in dioxane and methanol have been studied. Its fluorescence is bright in dioxane but faint in methanol and the photoisomerization in methanol is distinctly inefficient when compared with that in dioxane. By using time-resolved spectroscopy technology and quantum chemical calculation, hydrogen bonding strengthening between solutes and solvents in excited state, which induces enormously enhanced efficiency of internal conversion of HI in excited state, is assigned to the reason of significant fluorescence quenching and inefficient photoisomerization of HI in methanol. Because of the drastically fluorescence quenching effect of HI, trace water sensing with a limit of detection of 10 ppm in dioxane suggests the potential of HI as a fluorescence probe to quantitate the water content in aprotic organic solvents.
中文翻译:

半靛蓝的光致激发态氢键增强用于质子溶剂中的剧烈荧光猝灭和非质子溶剂中的水传感
摘要 激发态氢键可以调控有机分子的光化学和光物理过程。在这项工作中,研究了半靛蓝 (HI) 在二恶烷和甲醇中的荧光和光异构化特性。其荧光在二恶烷中较亮,在甲醇中较弱,与二恶烷相比,甲醇中的光异构化效率明显低下。利用时间分辨光谱技术和量子化学计算,激发态溶质与溶剂之间的氢键增强,导致激发态 HI 的内转化效率大大提高,被认为是荧光猝灭显着和光异构化效率低下的原因。甲醇中的 HI。由于 HI 的强烈荧光猝灭效应,
更新日期:2020-04-01
中文翻译:

半靛蓝的光致激发态氢键增强用于质子溶剂中的剧烈荧光猝灭和非质子溶剂中的水传感
摘要 激发态氢键可以调控有机分子的光化学和光物理过程。在这项工作中,研究了半靛蓝 (HI) 在二恶烷和甲醇中的荧光和光异构化特性。其荧光在二恶烷中较亮,在甲醇中较弱,与二恶烷相比,甲醇中的光异构化效率明显低下。利用时间分辨光谱技术和量子化学计算,激发态溶质与溶剂之间的氢键增强,导致激发态 HI 的内转化效率大大提高,被认为是荧光猝灭显着和光异构化效率低下的原因。甲醇中的 HI。由于 HI 的强烈荧光猝灭效应,




















































 京公网安备 11010802027423号
京公网安备 11010802027423号