当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalization of Piperidine Derivatives for the Site-Selective and Stereoselective Synthesis of Positional Analogues of Methylphenidate.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-09 , DOI: 10.1002/chem.201905773 Wenbin Liu 1 , Tobias Babl 1, 2 , Alexander Röther 2 , Oliver Reiser 2 , Huw M L Davies 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-09 , DOI: 10.1002/chem.201905773 Wenbin Liu 1 , Tobias Babl 1, 2 , Alexander Röther 2 , Oliver Reiser 2 , Huw M L Davies 1
Affiliation
|
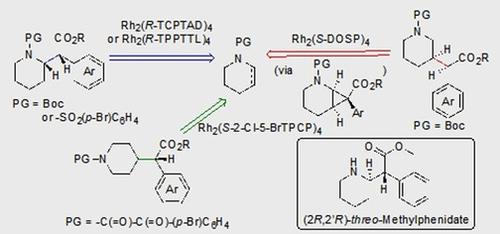
Rhodium-catalyzed C-H insertions and cyclopropanations of donor/acceptor carbenes have been used for the synthesis of positional analogues of methylphenidate. The site selectivity is controlled by the catalyst and the amine protecting group. C-H functionalization of N-Boc-piperidine using Rh2 (R-TCPTAD)4 , or N-brosyl-piperidine using Rh2 (R-TPPTTL)4 generated 2-substitited analogues. In contrast, when N-α-oxoarylacetyl-piperidines were used in combination with Rh2 (S-2-Cl-5-BrTPCP)4 , the C-H functionalization produced 4-susbstiuted analogues. Finally, the 3-substituted analogues were prepared indirectly by cyclopropanation of N-Boc-tetrahydropyridine followed by reductive regio- and stereoselective ring-opening of the cyclopropanes.
中文翻译:

哌啶衍生物的功能化用于定点和立体选择性合成哌醋甲酯的位置类似物。
铑催化的供体/受体碳烯的CH插入和环丙烷化已用于合成哌醋甲酯的位置类似物。位置选择性由催化剂和胺保护基控制。使用Rh2(R-TCPTAD)4的N-Boc-哌啶的CH官能化,或使用Rh2(R-TPPTTL)4的N-溴化哌啶的CH官能化生成2取代的类似物。相反,当将N-α-氧代芳基乙酰基-哌啶与Rh2(S-2-Cl-5-BrTPCP)4组合使用时,CH官能化会产生4位被怀疑的类似物。最后,通过N-Boc-四氢吡啶的环丙烷化,然后还原环丙烷的立体和立体选择性开环,间接制备3-取代的类似物。
更新日期:2020-03-09
中文翻译:

哌啶衍生物的功能化用于定点和立体选择性合成哌醋甲酯的位置类似物。
铑催化的供体/受体碳烯的CH插入和环丙烷化已用于合成哌醋甲酯的位置类似物。位置选择性由催化剂和胺保护基控制。使用Rh2(R-TCPTAD)4的N-Boc-哌啶的CH官能化,或使用Rh2(R-TPPTTL)4的N-溴化哌啶的CH官能化生成2取代的类似物。相反,当将N-α-氧代芳基乙酰基-哌啶与Rh2(S-2-Cl-5-BrTPCP)4组合使用时,CH官能化会产生4位被怀疑的类似物。最后,通过N-Boc-四氢吡啶的环丙烷化,然后还原环丙烷的立体和立体选择性开环,间接制备3-取代的类似物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号