当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Platinum Nanozyme Immobilized on 2D Metal-Organic Frameworks for Mitochondrion-Targeting and Oxygen Self-Supply Photodynamic Therapy.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-02 , DOI: 10.1021/acsami.9b14958 Zhiguo Gao 1 , Yaojia Li 1 , Yu Zhang 1 , Kaiwu Cheng 1 , Peijing An 1 , Fanghui Chen 1 , Jian Chen 1 , Chaoqun You 2 , Qing Zhu 3 , Baiwang Sun 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-02 , DOI: 10.1021/acsami.9b14958 Zhiguo Gao 1 , Yaojia Li 1 , Yu Zhang 1 , Kaiwu Cheng 1 , Peijing An 1 , Fanghui Chen 1 , Jian Chen 1 , Chaoqun You 2 , Qing Zhu 3 , Baiwang Sun 1
Affiliation
|
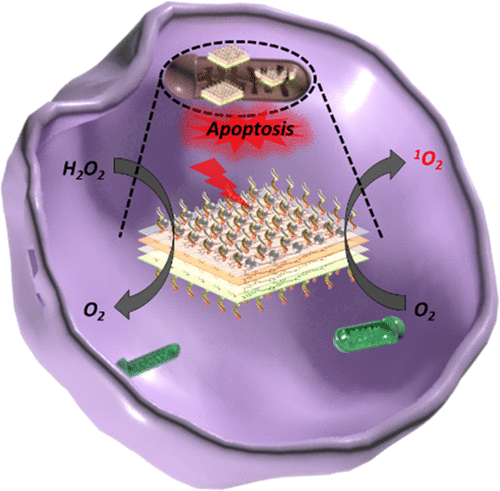
Photodynamic therapy (PDT) as a noninvasive therapy mode has attracted considerable attention in the field of oncotherapy. However, the PDT efficacy is restricted either by the tumor hypoxia environment or the inherent properties of photosensitizers (PSs) including bad water solution, photobleaching, and easy aggregation. Herein, we designed and synthesized a new two-dimensional (2D) metal-organic framework, Sm-tetrakis(4-carboxyphenyl)porphyrin (TCPP) nanosheets, by assembling transition metal ions (Sm3+) and PSs (TCPP), on which the catalase (CAT)-mimicking platinum nanozymes were then in situ grown for sufficient oxygen supply during PDT. The prepared Sm-TCPP with nanoplate morphology (∼100 nm in diameter) and ultrathin thickness (<10 nm) showed significantly enhanced 1O2 generation capacity due to the improved physicochemical properties and the enhanced intersystem crossing from heavy Sm nodes. More importantly, the CAT-mimicking Pt nanozyme on the Sm-TCPP nanosheets could effectively convert over-expressed H2O2 in the tumor microenvironment into O2 to relieve tumor hypoxia. Further, the triphenylphosphine (TPP) molecule was introduced to Sm-TCPP-Pt to develop a mitochondrion-targeting and O2 self-supply PDT system. The in vitro and in vivo experimental results based on the MCF-7 breast cancer model revealed that Sm-TCPP-Pt/TPP could relieve tumor hypoxia and the generated reactive oxygen species nearby intracellular mitochondria significantly induced cell apoptosis. This study offers an engineering strategy to integrate 2D PS-based metal-organic frameworks and nanozymes into a nanoplatform to surmount the pitfalls of traditional PDT.
中文翻译:

仿生铂纳米酶固定在2D金属有机骨架上,用于线粒体靶向和氧气自给式光动力疗法。
光动力疗法(PDT)作为一种非侵入性治疗模式已在肿瘤治疗领域引起了相当大的关注。但是,PDT的功效受到肿瘤缺氧环境或光敏剂(PSs)固有特性(包括不良水溶液,光致漂白和易于聚集)的限制。在这里,我们通过组装过渡金属离子(Sm3 +)和PSs(TCPP),设计并合成了一种新的二维(2D)金属有机框架Sm-四(4-羧基苯基)卟啉(TCPP)纳米片。然后,原位生长类似于过氧化氢酶(CAT)的铂纳米酶,以便在PDT期间提供足够的氧气。制备的Sm-TCPP具有纳米板形态(直径约100 nm)和超薄厚度(< 10 nm)表现出显着增强的1O2生成能力,这是由于改善了的理化特性和增强了与重Sm节点的系统间交叉。更重要的是,Sm-TCPP纳米片上模仿CAT的Pt纳米酶可以有效地将肿瘤微环境中过表达的H2O2转化为O2,以缓解肿瘤的缺氧。此外,将三苯基膦(TPP)分子引入Sm-TCPP-Pt,以开发线粒体靶向和O2自给的PDT系统。基于MCF-7乳腺癌模型的体内外实验结果表明,Sm-TCPP-Pt / TPP可以缓解肿瘤缺氧,细胞内线粒体附近产生的活性氧显着诱导细胞凋亡。
更新日期:2020-01-02
中文翻译:

仿生铂纳米酶固定在2D金属有机骨架上,用于线粒体靶向和氧气自给式光动力疗法。
光动力疗法(PDT)作为一种非侵入性治疗模式已在肿瘤治疗领域引起了相当大的关注。但是,PDT的功效受到肿瘤缺氧环境或光敏剂(PSs)固有特性(包括不良水溶液,光致漂白和易于聚集)的限制。在这里,我们通过组装过渡金属离子(Sm3 +)和PSs(TCPP),设计并合成了一种新的二维(2D)金属有机框架Sm-四(4-羧基苯基)卟啉(TCPP)纳米片。然后,原位生长类似于过氧化氢酶(CAT)的铂纳米酶,以便在PDT期间提供足够的氧气。制备的Sm-TCPP具有纳米板形态(直径约100 nm)和超薄厚度(< 10 nm)表现出显着增强的1O2生成能力,这是由于改善了的理化特性和增强了与重Sm节点的系统间交叉。更重要的是,Sm-TCPP纳米片上模仿CAT的Pt纳米酶可以有效地将肿瘤微环境中过表达的H2O2转化为O2,以缓解肿瘤的缺氧。此外,将三苯基膦(TPP)分子引入Sm-TCPP-Pt,以开发线粒体靶向和O2自给的PDT系统。基于MCF-7乳腺癌模型的体内外实验结果表明,Sm-TCPP-Pt / TPP可以缓解肿瘤缺氧,细胞内线粒体附近产生的活性氧显着诱导细胞凋亡。

































 京公网安备 11010802027423号
京公网安备 11010802027423号