当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Variation in the In2O3 Crystal Phase Alters Catalytic Performance toward the Reverse Water Gas Shift Reaction
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-21 , DOI: 10.1021/acscatal.9b04239 Jianyang Wang 1 , Chun-Yen Liu 2 , Thomas P. Senftle 2 , Jie Zhu 1 , Guanghui Zhang 1 , Xinwen Guo 1 , Chunshan Song 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-21 , DOI: 10.1021/acscatal.9b04239 Jianyang Wang 1 , Chun-Yen Liu 2 , Thomas P. Senftle 2 , Jie Zhu 1 , Guanghui Zhang 1 , Xinwen Guo 1 , Chunshan Song 1, 3
Affiliation
|
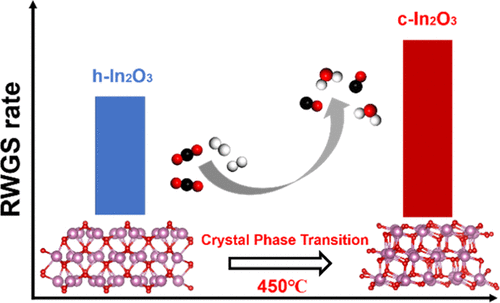
Understanding the structure–catalytic activity relationship is crucial for developing new catalysts with desired performance. In this contribution, we report the performance of In2O3 with different crystal phases in the reverse water gas shift (RWGS) reaction, where we observe changing activity induced by a phase transition under reaction conditions. Cubic In2O3 (c-In2O3) exhibits a higher RWGS rate than the hexagonal phase (h-In2O3) at temperatures below 350 °C because of its (1) enhanced dissociative adsorption of H2, (2) facile formation of the oxygen vacancies, and (3) enhanced ability to adsorb and activate CO2 on the oxygen vacancies, as suggested both experimentally and computationally. Density functional theory results indicate that the surface oxygen arrangement on the cubic polymorph is key to rapid H2 adsorption, which facilitates oxygen vacancy formation and subsequent CO2 adsorption to yield high RWGS reactivity. At 450 °C and above, the activity of h-In2O3 increases gradually with time on stream, which is caused by a phase transition from h-In2O3 to c-In2O3. In situ X-ray diffraction experiments show that h-In2O3 is first reduced by H2 and subsequently oxidized by CO2 to c-In2O3. These findings highlight the importance of the crystal phase in the catalytic RWGS reaction and provide a new dimension for understanding/designing RWGS catalysts.
中文翻译:

In 2 O 3晶相的变化改变了对水煤气逆反应的催化性能
了解结构与催化活性之间的关系对于开发具有所需性能的新型催化剂至关重要。在这一贡献中,我们报告了在反向水煤气变换(RWGS)反应中具有不同晶相的In 2 O 3的性能,在该反应中我们观察到在反应条件下由相变引起的活性变化。在低于350°C的温度下,立方In 2 O 3(c-In 2 O 3)具有比六方相(h-In 2 O 3)更高的RWGS速率,这是因为其(1)增强了H 2的解离吸附,(2)容易形成氧空位,以及(3)增强了在氧空位上吸附和激活CO 2的能力,这在实验和计算上都表明了这一点。密度泛函理论结果表明,立方多晶型物上的表面氧排列是快速吸附H 2的关键,这有助于氧空位的形成和随后的CO 2吸附,从而产生较高的RWGS反应性。在450°C和更高温度下,h-In 2 O 3的活性会随着运行时间的增加而逐渐增加,这是由于从h-In 2 O 3到c-In 2 O 3的相变而引起的。。原位X射线衍射实验表明,h-In 2 O 3首先被H 2还原,然后被CO 2氧化成c-In 2 O 3。这些发现突出了结晶相在催化RWGS反应中的重要性,并为理解/设计RWGS催化剂提供了新的视角。
更新日期:2020-02-21
中文翻译:

In 2 O 3晶相的变化改变了对水煤气逆反应的催化性能
了解结构与催化活性之间的关系对于开发具有所需性能的新型催化剂至关重要。在这一贡献中,我们报告了在反向水煤气变换(RWGS)反应中具有不同晶相的In 2 O 3的性能,在该反应中我们观察到在反应条件下由相变引起的活性变化。在低于350°C的温度下,立方In 2 O 3(c-In 2 O 3)具有比六方相(h-In 2 O 3)更高的RWGS速率,这是因为其(1)增强了H 2的解离吸附,(2)容易形成氧空位,以及(3)增强了在氧空位上吸附和激活CO 2的能力,这在实验和计算上都表明了这一点。密度泛函理论结果表明,立方多晶型物上的表面氧排列是快速吸附H 2的关键,这有助于氧空位的形成和随后的CO 2吸附,从而产生较高的RWGS反应性。在450°C和更高温度下,h-In 2 O 3的活性会随着运行时间的增加而逐渐增加,这是由于从h-In 2 O 3到c-In 2 O 3的相变而引起的。。原位X射线衍射实验表明,h-In 2 O 3首先被H 2还原,然后被CO 2氧化成c-In 2 O 3。这些发现突出了结晶相在催化RWGS反应中的重要性,并为理解/设计RWGS催化剂提供了新的视角。































 京公网安备 11010802027423号
京公网安备 11010802027423号