当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis for ribosome recycling by RRF and tRNA.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41594-019-0350-7 Dejian Zhou 1 , Takehito Tanzawa 2 , Jinzhong Lin 1 , Matthieu G Gagnon 2, 3, 4
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41594-019-0350-7 Dejian Zhou 1 , Takehito Tanzawa 2 , Jinzhong Lin 1 , Matthieu G Gagnon 2, 3, 4
Affiliation
|
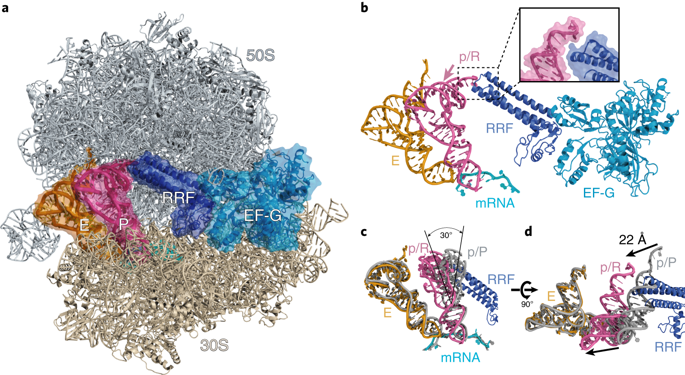
The bacterial ribosome is recycled into subunits by two conserved proteins, elongation factor G (EF-G) and the ribosome recycling factor (RRF). The molecular basis for ribosome recycling by RRF and EF-G remains unclear. Here, we report the crystal structure of a posttermination Thermus thermophilus 70S ribosome complexed with EF-G, RRF and two transfer RNAs at a resolution of 3.5 Å. The deacylated tRNA in the peptidyl (P) site moves into a previously unsuspected state of binding (peptidyl/recycling, p/R) that is analogous to that seen during initiation. The terminal end of the p/R-tRNA forms nonfavorable contacts with the 50S subunit while RRF wedges next to central inter-subunit bridges, illuminating the active roles of tRNA and RRF in dissociation of ribosomal subunits. The structure uncovers a missing snapshot of tRNA as it transits between the P and exit (E) sites, providing insights into the mechanisms of ribosome recycling and tRNA translocation.
中文翻译:

RRF和tRNA再循环核糖体的结构基础。
细菌核糖体被两种保守的蛋白质、延伸因子 G (EF-G) 和核糖体回收因子 (RRF) 回收成亚基。RRF 和 EF-G 进行核糖体循环的分子基础仍不清楚。在这里,我们报告了与 EF-G、RRF 和两个转移 RNA 复合的终止后嗜热栖热菌 70S 核糖体的晶体结构,分辨率为 3.5 Å。肽基 (P) 位点中的脱酰基 tRNA 进入以前未预料到的结合状态(肽基/循环,p/R),这与起始期间看到的类似。p/R-tRNA 的末端与 50S 亚基形成不利接触,而 RRF 楔入中央亚基间桥旁边,说明 tRNA 和 RRF 在核糖体亚基解离中的积极作用。
更新日期:2019-12-23
中文翻译:

RRF和tRNA再循环核糖体的结构基础。
细菌核糖体被两种保守的蛋白质、延伸因子 G (EF-G) 和核糖体回收因子 (RRF) 回收成亚基。RRF 和 EF-G 进行核糖体循环的分子基础仍不清楚。在这里,我们报告了与 EF-G、RRF 和两个转移 RNA 复合的终止后嗜热栖热菌 70S 核糖体的晶体结构,分辨率为 3.5 Å。肽基 (P) 位点中的脱酰基 tRNA 进入以前未预料到的结合状态(肽基/循环,p/R),这与起始期间看到的类似。p/R-tRNA 的末端与 50S 亚基形成不利接触,而 RRF 楔入中央亚基间桥旁边,说明 tRNA 和 RRF 在核糖体亚基解离中的积极作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号