当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed ortho-Metalation and Anionic ortho-Fries Rearrangement of Polycyclic Aromatic O-Carbamates: Regioselective Synthesis of Substituted Chrysenes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acs.joc.7b03210 Sindhu Kancherla 1 , Marianne Lorentzen 1 , Victor Snieckus 2 , Kåre B. Jørgensen 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acs.joc.7b03210 Sindhu Kancherla 1 , Marianne Lorentzen 1 , Victor Snieckus 2 , Kåre B. Jørgensen 1
Affiliation
|
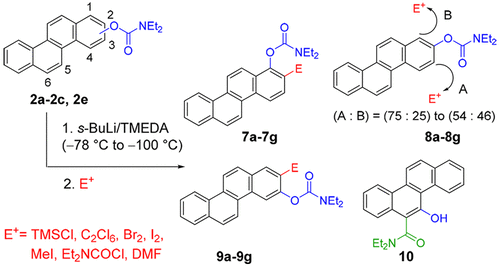
A general method for the regioselective synthesis of a series of ortho-substituted chrysenyl N,N-diethyl-O-carbamates by the directed ortho-metalation (DoM) strategy is reported. The starting O-carbamates were prepared from the corresponding chrysenols, available by oxidative photochemical cyclization or directed remote metalation tactics. Chrysen-1-yl and chrysene-3-yl ring site selectivity of directed ortho-metalation (DoM) and anionic ortho-Fries rearrangement (AoF) protocols, with s-BuLi/TMEDA, followed by electrophilic quench using a selection of electrophiles, were observed, leading to new chrysenyl derivatives. 5-Chrysenyl N,N-diethyl-O-carbamate underwent instant AoF rearrangement even at −100 °C to furnish chrysenyl o-hydroxycarboxamide. Iterative DoM reactions were carried out to gain insight into the regioselectivity factors.
中文翻译:

定向邻-Metalation和阴离子邻多环芳烃-Fries重排Ø -Carbamates:替补Chrysenes的区域选择性合成
报道了通过定向邻位金属化(D o M)策略区域选择性合成一系列邻位取代的N,N-二乙基-O-氨基甲酸酯的一般方法。起始的O-氨基甲酸酯是由相应的酚类化合物制备的,可以通过氧化光化学环化或直接的远程金属化策略获得。屈(chrysene)哌啶-1-基和苯并菲-3-基环部位选择性定向邻-metalation(d Ò M)和阴离子邻-Fries重排(A ö F)协议,与小号观察到-BuLi / TMEDA,随后使用选择的亲电试剂进行亲电淬灭,产生了新的Chrysenyl衍生物。5屈基N,N-二乙基ø -氨基甲酸酯后行瞬间甲ö ˚F即使在-100℃至配料屈重排Ò羟基甲酰胺。进行了反复的D o M反应,以了解区域选择性因素。
更新日期:2018-03-15
中文翻译:

定向邻-Metalation和阴离子邻多环芳烃-Fries重排Ø -Carbamates:替补Chrysenes的区域选择性合成
报道了通过定向邻位金属化(D o M)策略区域选择性合成一系列邻位取代的N,N-二乙基-O-氨基甲酸酯的一般方法。起始的O-氨基甲酸酯是由相应的酚类化合物制备的,可以通过氧化光化学环化或直接的远程金属化策略获得。屈(chrysene)哌啶-1-基和苯并菲-3-基环部位选择性定向邻-metalation(d Ò M)和阴离子邻-Fries重排(A ö F)协议,与小号观察到-BuLi / TMEDA,随后使用选择的亲电试剂进行亲电淬灭,产生了新的Chrysenyl衍生物。5屈基N,N-二乙基ø -氨基甲酸酯后行瞬间甲ö ˚F即使在-100℃至配料屈重排Ò羟基甲酰胺。进行了反复的D o M反应,以了解区域选择性因素。





























 京公网安备 11010802027423号
京公网安备 11010802027423号