当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Ripostatin B and Structure–Activity Relationship Studies on Ripostatin Analogs
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acs.joc.8b00193 Florian Glaus 1 , Darija Dedić 1 , Priyanka Tare 2 , Valakunja Nagaraja 2 , Liliana Rodrigues 3, 4 , José Antonio Aínsa 3 , Jens Kunze 1 , Gisbert Schneider 1 , Ruben C. Hartkoorn 5 , Stewart T. Cole 5 , Karl-Heinz Altmann 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acs.joc.8b00193 Florian Glaus 1 , Darija Dedić 1 , Priyanka Tare 2 , Valakunja Nagaraja 2 , Liliana Rodrigues 3, 4 , José Antonio Aínsa 3 , Jens Kunze 1 , Gisbert Schneider 1 , Ruben C. Hartkoorn 5 , Stewart T. Cole 5 , Karl-Heinz Altmann 1
Affiliation
|
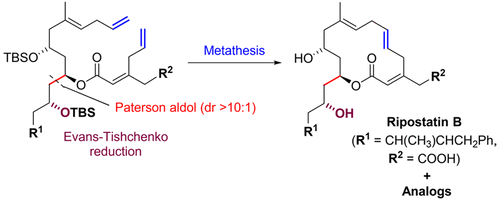
Described is the total synthesis of the myxobacterial natural product ripostatin B and of a small number of analogs. Ripostatin B is a polyketide-derived 14-membered macrolide that acts as an inhibitor of bacterial RNA-polymerase, but is mechanistically distinct from rifamycin-derived RNA-polymerase inhibitors that are in use for tuberculosis treatment. The macrolactone ring of ripostatin B features two stereocenters and a synthetically challenging doubly skipped triene motif, with one of the double bonds being in conjugation with the ester carbonyl. Appended to the macrolactone core are an extended hydroxy-bearing phenylalkyl side chain at C13 and a carboxymethyl group at C3. The triene motif was established with high efficiency by ring-closing olefin metathesis, which proceeded in almost 80% yield. The side chain-bearing stereocenter α to the ester oxygen was formed in a Paterson aldol reaction between a methyl ketone and a β-chiral β-hydroxy aldehyde with excellent syn selectivity (dr >10:1). The total synthesis provided a blueprint for the synthesis of analogs with modifications in the C3 and C13 side chains. The C3-modified analogs showed good antibacterial activity against efflux-deficient Escherichia coli but, as ripostatin B, were inactive against Mycobacterium tuberculosis, in spite of significant in vitro inhibition of M. tuberculosis RNA-polymerase.
中文翻译:

Ripostatin B的全合成及Ripostatin类似物的构效关系研究
描述了粘细菌天然产物利波西汀B和少量类似物的总合成。Ripostatin B是一种聚酮化合物衍生的14元大环内酯类化合物,可作为细菌RNA聚合酶的抑制剂,但在机理上不同于用于结核病治疗的利福霉素衍生的RNA聚合酶抑制剂。ripostatin B的大内酯环具有两个立体中心和一个具有挑战性的双跳三烯基序,双键之一与酯羰基共轭。与大内酯核连接的是在C13处带有羟基的延伸的苯基烷基侧链和在C3处具有羧甲基的基团。通过闭环烯烃复分解高效地建立了三烯基序,该复分解反应以近80%的收率进行。syn选择性(dr> 10:1)。总合成为合成具有C3和C13侧链的类似物提供了一个蓝图。经过C3修饰的类似物对排泄缺陷的大肠杆菌表现出良好的抗菌活性,但是,尽管有明显的体外抑制结核分枝杆菌RNA聚合酶的作用,但如ripostatin B一样,它对结核分枝杆菌没有活性。
更新日期:2018-03-15
中文翻译:

Ripostatin B的全合成及Ripostatin类似物的构效关系研究
描述了粘细菌天然产物利波西汀B和少量类似物的总合成。Ripostatin B是一种聚酮化合物衍生的14元大环内酯类化合物,可作为细菌RNA聚合酶的抑制剂,但在机理上不同于用于结核病治疗的利福霉素衍生的RNA聚合酶抑制剂。ripostatin B的大内酯环具有两个立体中心和一个具有挑战性的双跳三烯基序,双键之一与酯羰基共轭。与大内酯核连接的是在C13处带有羟基的延伸的苯基烷基侧链和在C3处具有羧甲基的基团。通过闭环烯烃复分解高效地建立了三烯基序,该复分解反应以近80%的收率进行。syn选择性(dr> 10:1)。总合成为合成具有C3和C13侧链的类似物提供了一个蓝图。经过C3修饰的类似物对排泄缺陷的大肠杆菌表现出良好的抗菌活性,但是,尽管有明显的体外抑制结核分枝杆菌RNA聚合酶的作用,但如ripostatin B一样,它对结核分枝杆菌没有活性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号