当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic C(sp3)-H bond activation in tertiary alkylamines.
Nature Chemistry ( IF 19.2 ) Pub Date : 2019-12-20 , DOI: 10.1038/s41557-019-0393-8 Jesus Rodrigalvarez 1 , Manuel Nappi 1 , Hiroki Azuma 1 , Nils J Flodén 1 , Matthew E Burns 1 , Matthew J Gaunt 1
Nature Chemistry ( IF 19.2 ) Pub Date : 2019-12-20 , DOI: 10.1038/s41557-019-0393-8 Jesus Rodrigalvarez 1 , Manuel Nappi 1 , Hiroki Azuma 1 , Nils J Flodén 1 , Matthew E Burns 1 , Matthew J Gaunt 1
Affiliation
|
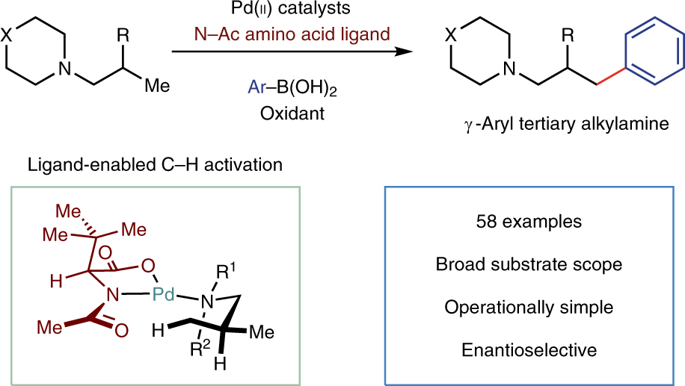
The development of robust catalytic methods to assemble tertiary alkylamines provides a continual challenge to chemical synthesis. In this regard, transformation of a traditionally unreactive C-H bond, proximal to the nitrogen atom, into a versatile chemical entity would be a powerful strategy for introducing functional complexity to tertiary alkylamines. A practical and selective metal-catalysed C(sp3)-H activation facilitated by the tertiary alkylamine functionality, however, remains an unsolved problem. Here, we report a Pd(II)-catalysed protocol that appends arene feedstocks to tertiary alkylamines via C(sp3)-H functionalization. A simple ligand for Pd(II) orchestrates the C-H activation step in favour of deleterious pathways. The reaction can use both simple and complex starting materials to produce a range of multifaceted γ-aryl tertiary alkylamines and can be rendered enantioselective. The enabling features of this transformation should be attractive to practitioners of synthetic and medicinal chemistry as well as in other areas that use biologically active alkylamines.
中文翻译:

叔烷基胺中的催化C(sp3)-H键活化。
组装叔烷基胺的鲁棒催化方法的发展为化学合成提供了持续的挑战。在这方面,将接近氮原子的传统上不反应的CH键转变为通用的化学实体将是一种将功能复杂性引入叔烷基胺的有效策略。然而,由叔烷基胺官能团促进的实用的和选择性的金属催化的C(sp3)-H活化仍然是一个未解决的问题。在这里,我们报告Pd(II)催化的协议,将芳烃原料通过C(sp3)-H官能化附加到叔烷基胺上。Pd(II)的一个简单配体协调CH活化步骤,有利于有害途径。该反应既可以使用简单的原料也可以使用复杂的原料,以产生一系列多面的γ-芳基叔烷基胺,并且可以被对映选择性。这种转变的有利特征应该对合成化学和药物化学的从业者以及使用生物活性烷基胺的其他领域具有吸引力。
更新日期:2019-12-21
中文翻译:

叔烷基胺中的催化C(sp3)-H键活化。
组装叔烷基胺的鲁棒催化方法的发展为化学合成提供了持续的挑战。在这方面,将接近氮原子的传统上不反应的CH键转变为通用的化学实体将是一种将功能复杂性引入叔烷基胺的有效策略。然而,由叔烷基胺官能团促进的实用的和选择性的金属催化的C(sp3)-H活化仍然是一个未解决的问题。在这里,我们报告Pd(II)催化的协议,将芳烃原料通过C(sp3)-H官能化附加到叔烷基胺上。Pd(II)的一个简单配体协调CH活化步骤,有利于有害途径。该反应既可以使用简单的原料也可以使用复杂的原料,以产生一系列多面的γ-芳基叔烷基胺,并且可以被对映选择性。这种转变的有利特征应该对合成化学和药物化学的从业者以及使用生物活性烷基胺的其他领域具有吸引力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号