当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Universal Affinity Capture Liquid Chromatography-Mass Spectrometry Assay for Evaluation of Biotransformation of Site-Specific Antibody Drug Conjugates in Preclinical Studies.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.analchem.9b04572 Srikanth Kotapati , David Passmore , Sayumi Yamazoe , Rajesh Kishore Kumar Sanku , Qiang Cong , Yam B. Poudel , Naidu S. Chowdari , Sanjeev Gangwar , Chetana Rao , Vangipuram S. Rangan , Pina M. Cardarelli , Shrikant Deshpande , Pavel Strop , Gavin Dollinger , Arvind Rajpal
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.analchem.9b04572 Srikanth Kotapati , David Passmore , Sayumi Yamazoe , Rajesh Kishore Kumar Sanku , Qiang Cong , Yam B. Poudel , Naidu S. Chowdari , Sanjeev Gangwar , Chetana Rao , Vangipuram S. Rangan , Pina M. Cardarelli , Shrikant Deshpande , Pavel Strop , Gavin Dollinger , Arvind Rajpal
|
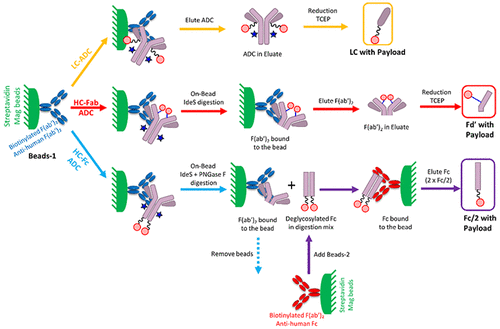
Antibody drug conjugates (ADCs) can undergo in vivo biotransformation (e.g., payload metabolism, deconjugation) leading to reduced or complete loss of activity. The location/site of conjugation of payload-linker can have an effect on ADC stability and hence needs to be carefully optimized. Affinity capture LC-MS of intact ADCs or ADC subfragments has been extensively used to evaluate ADC biotransformation. However, the current methods have certain limitations such as the requirement of specific capture reagents, limited mass resolution of low mass change metabolites, low sensitivity, and use of capillary or nanoflow LC-MS. To address these challenges, we developed a generic affinity capture LC-MS assay that can be utilized to evaluate the biotransformation of any site-specific ADC independent of antibody type and site of conjugation (Fab and Fc) in preclinical studies. The method involves a combination of some or all of these steps: (1) "mono capture" or "dual capture" of ADCs from serum with streptavidin magnetic beads coated with a generic biotinylated antihuman capture reagent, (2) "on-bead" digestion with IdeS and/or PNGase F, and (3) reduction of interchain disulfide bonds to generate ∼25 kDa ADC subfragments, which are finally analyzed by LC-HRMS on a TOF mass spectrometer. The advantages of this method are that it can be performed using commercially available generic reagents and requires sample preparation time of less than 7 h. Furthermore, by reducing the size of intact ADC (∼150 kDa) to subfragments (∼25 kDa), the identification of conjugated payload and its metabolites can be achieved with excellent sensitivity and resolution (hydrolysis and other small mass change metabolites). This method was successfully applied to evaluate the in vitro and in vivo biotransformation of ADCs conjugated at different sites (LC, HC-Fab, and HC-Fc) with various classes of payload-linkers.
中文翻译:

临床前研究中用于评估特定部位抗体药物结合物生物转化的通用亲和捕获液相色谱-质谱分析法。
抗体药物偶联物(ADC)可以进行体内生物转化(例如有效载荷代谢,去偶联),从而导致活性降低或完全丧失。有效负载链接器的共轭位置/位置可能会影响ADC的稳定性,因此需要仔细优化。完整ADC或ADC亚片段的亲和捕获LC-MS已被广泛用于评估ADC的生物转化。但是,当前的方法具有某些局限性,例如需要特定的捕获试剂,质量变化较小的代谢物的质量分辨率有限,灵敏度较低以及使用毛细管或纳流LC-MS。为了应对这些挑战,我们开发了一种通用的亲和捕获LC-MS分析方法,可用于评估临床前研究中与抗体类型和结合位点(Fab和Fc)无关的任何位点特异性ADC的生物转化。该方法涉及这些步骤中的一些或全部步骤的组合:(1)用涂有通用生物素化抗人类捕获试剂的抗生蛋白链菌素磁珠从血清中“单捕获”或“双重捕获” ADC,(2)“在珠子上”用IdeS和/或PNGase F酶切,(3)还原链间二硫键,生成〜25 kDa ADC亚片段,最后用TOF质谱仪通过LC-HRMS分析。该方法的优点是可以使用市售的通用试剂进行,并且样品制备时间少于7小时。此外,通过将完整ADC的大小(〜150 kDa)减小到亚片段(〜25 kDa),可以以出色的灵敏度和分辨率(水解和其他小的质量变化代谢物)实现共轭有效负载及其代谢物的鉴定。该方法已成功应用于评估在不同位点(LC,HC-Fab和HC-Fc)上缀合有各种有效负载连接子的ADC的体外和体内生物转化。
更新日期:2020-01-07
中文翻译:

临床前研究中用于评估特定部位抗体药物结合物生物转化的通用亲和捕获液相色谱-质谱分析法。
抗体药物偶联物(ADC)可以进行体内生物转化(例如有效载荷代谢,去偶联),从而导致活性降低或完全丧失。有效负载链接器的共轭位置/位置可能会影响ADC的稳定性,因此需要仔细优化。完整ADC或ADC亚片段的亲和捕获LC-MS已被广泛用于评估ADC的生物转化。但是,当前的方法具有某些局限性,例如需要特定的捕获试剂,质量变化较小的代谢物的质量分辨率有限,灵敏度较低以及使用毛细管或纳流LC-MS。为了应对这些挑战,我们开发了一种通用的亲和捕获LC-MS分析方法,可用于评估临床前研究中与抗体类型和结合位点(Fab和Fc)无关的任何位点特异性ADC的生物转化。该方法涉及这些步骤中的一些或全部步骤的组合:(1)用涂有通用生物素化抗人类捕获试剂的抗生蛋白链菌素磁珠从血清中“单捕获”或“双重捕获” ADC,(2)“在珠子上”用IdeS和/或PNGase F酶切,(3)还原链间二硫键,生成〜25 kDa ADC亚片段,最后用TOF质谱仪通过LC-HRMS分析。该方法的优点是可以使用市售的通用试剂进行,并且样品制备时间少于7小时。此外,通过将完整ADC的大小(〜150 kDa)减小到亚片段(〜25 kDa),可以以出色的灵敏度和分辨率(水解和其他小的质量变化代谢物)实现共轭有效负载及其代谢物的鉴定。该方法已成功应用于评估在不同位点(LC,HC-Fab和HC-Fc)上缀合有各种有效负载连接子的ADC的体外和体内生物转化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号