当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expansion of chemical space based on a pyrrolo[1,2-a]pyrazine core: Synthesis and its anticancer activity in prostate cancer and breast cancer cells.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.ejmech.2019.111988 Yohan Seo 1 , Jeong Hwa Lee 1 , So-Hyeon Park 2 , Wan Namkung 3 , Ikyon Kim 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.ejmech.2019.111988 Yohan Seo 1 , Jeong Hwa Lee 1 , So-Hyeon Park 2 , Wan Namkung 3 , Ikyon Kim 1
Affiliation
|
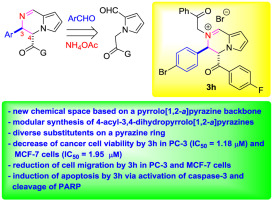
In connection with our continued research to generate new aza-fused heteroaromatic chemical scaffolds, we developed a highly atom-economical three-component route to novel 3,4-dihydropyrrolo[1,2-a]pyrazine ring skeleton multi-functionalized on the pyrazine unit. This [4+1+1] annulation approach led us to gain access to a new N-fused bicyclic chemical space having two distinctive functional groups (heteroaryl and aroyl) in a trans manner. Investigation of anticancer activity of the synthesized compounds and their derivatives revealed that (3R*,4S*)-3-(4-bromophenyl)-4-(4-fluorobenzoyl)-2-(2-oxo-2-phenylethyl)-3,4-dihydropyrrolo[1,2-a]pyrazin-2-ium bromide (3h) has potent anticancer activity. 3h significantly inhibited cell viability in prostate cancer cells (PC-3) and breast cancer cells (MCF-7) with IC50 value of 1.18 ± 0.05 μM and 1.95 ± 0.04 μM, respectively. In addition, 3h strongly reduced cell migration in a dose dependent manner, and induced apoptosis via caspase-3 activation and cleavage of PARP in PC-3 and MCF-7 cells. Our results in this study imply that 3h can be a potential anticancer agent against prostate cancer and breast cancer.
中文翻译:

基于吡咯并[1,2-a]吡嗪核心的化学空间的扩展:在前列腺癌和乳腺癌细胞中的合成及其抗癌活性。
与我们对产生新的氮杂稠合杂芳族化学支架的持续研究相结合,我们开发了一种高度原子经济的三组分途径,以在吡嗪上多功能化的新型3,4-二氢吡咯并[1,2-a]吡嗪环骨架单元。这种[4 + 1 + 1]环空方法使我们能够以反式方式进入具有两个独特官能团(杂芳基和芳酰基)的新的N稠合双环化学空间。合成化合物及其衍生物的抗癌活性研究表明,(3R *,4S *)-3-(4-溴苯基)-4-(4-氟苯甲酰基)-2-(2-氧代-2-苯基乙基)-3 ,4-二氢吡咯并[1,2-a]吡嗪-2-溴化铵(3h)具有有效的抗癌活性。3h显着抑制前列腺癌细胞(PC-3)和乳腺癌细胞(MCF-7)的细胞活力,IC50值分别为1.18±0.05μM和1.95±0.04μM,分别。此外,3h以剂量依赖性方式强烈减少细胞迁移,并通过caspase-3活化和PARP在PC-3和MCF-7细胞中的裂解诱导细胞凋亡。我们在这项研究中的结果表明3h可能是针对前列腺癌和乳腺癌的潜在抗癌药。
更新日期:2019-12-20
中文翻译:

基于吡咯并[1,2-a]吡嗪核心的化学空间的扩展:在前列腺癌和乳腺癌细胞中的合成及其抗癌活性。
与我们对产生新的氮杂稠合杂芳族化学支架的持续研究相结合,我们开发了一种高度原子经济的三组分途径,以在吡嗪上多功能化的新型3,4-二氢吡咯并[1,2-a]吡嗪环骨架单元。这种[4 + 1 + 1]环空方法使我们能够以反式方式进入具有两个独特官能团(杂芳基和芳酰基)的新的N稠合双环化学空间。合成化合物及其衍生物的抗癌活性研究表明,(3R *,4S *)-3-(4-溴苯基)-4-(4-氟苯甲酰基)-2-(2-氧代-2-苯基乙基)-3 ,4-二氢吡咯并[1,2-a]吡嗪-2-溴化铵(3h)具有有效的抗癌活性。3h显着抑制前列腺癌细胞(PC-3)和乳腺癌细胞(MCF-7)的细胞活力,IC50值分别为1.18±0.05μM和1.95±0.04μM,分别。此外,3h以剂量依赖性方式强烈减少细胞迁移,并通过caspase-3活化和PARP在PC-3和MCF-7细胞中的裂解诱导细胞凋亡。我们在这项研究中的结果表明3h可能是针对前列腺癌和乳腺癌的潜在抗癌药。































 京公网安备 11010802027423号
京公网安备 11010802027423号