当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calculations of pKa Values of Selected Pyridinium and Its N-Oxide Ions in Water and Acetonitrile.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jpca.9b10319
Paulina Mech 1 , Małgorzata Bogunia 1 , Andrzej Nowacki 1 , Mariusz Makowski 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jpca.9b10319
Paulina Mech 1 , Małgorzata Bogunia 1 , Andrzej Nowacki 1 , Mariusz Makowski 1
Affiliation
|
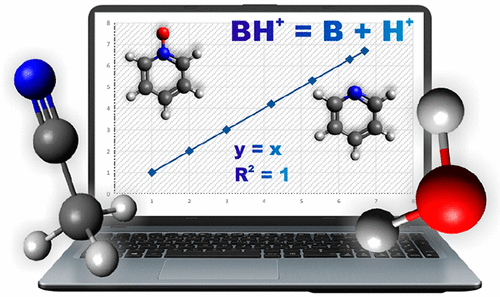
Pyridine, its N-oxide, and their derivatives are exciting classes of organic bases. These compounds show widespread biological activity, and they are often used in synthesis. In this work results on theoretical calculations of acid dissociation constants as pKa of pyridine, its N-oxide, and their derivatives were done based on the thermodynamic cycle in water and acetonitrile. Additionally, gas-phase basicity (GB) and proton affinity (PA) values were computed for systems studied. All pKa values were obtained using B3LYP, M06-2X, and G4MP2 methods in the gas phase, which were combined with the PCM model calculations (at the Hartree-Fock method) and with the use of four different scale factors alpha. Theoretical GB, PA, and pKa values were then compared with the available experimental ones. Results obtained from B3LYP and M06-2X methods are quite similar and compatible with experimental ones in terms of quality with correlation coefficients values R2 higher than 0.9, whereas results received from G4MP2 deviate strongly. The calculated pKa values are highly sensitive to the scale factors alpha used in the computational procedure. Root-mean-square deviations (RMSD) between both theoretically and experimentally available pKa values of systems studied were also computed. The RMSD values are lower than 0.8 for the best results, suggesting that the theoretical model presented in this work is promising for applications for pKa calculations of different classes of compounds.
中文翻译:

计算水中和乙腈中选定的吡啶及其氮氧化物的pKa值。
吡啶,其N氧化物及其衍生物是令人兴奋的有机碱。这些化合物具有广泛的生物活性,通常用于合成。在这项工作中,基于水和乙腈中的热力学循环,完成了对酸解离常数(如吡啶的pKa,其N-氧化物及其衍生物)的理论计算结果。此外,还针对所研究的系统计算了气相碱度(GB)和质子亲和力(PA)值。所有的pKa值都是在气相中使用B3LYP,M06-2X和G4MP2方法获得的,将它们与PCM模型计算(在Hartree-Fock方法中)相结合,并使用四个不同的比例因子alpha。然后将理论GB,PA和pKa值与可用的实验值进行比较。从B3LYP和M06-2X方法获得的结果在质量上非常相似,并且与实验结果兼容,相关系数值R2高于0.9,而从G4MP2接收的结果则有很大差异。计算出的pKa值对计算过程中使用的比例因子alpha高度敏感。还计算了所研究系统的理论和实验上可用的pKa值之间的均方根偏差(RMSD)。为了获得最佳结果,RMSD值低于0.8,这表明这项工作中提出的理论模型有望用于不同类别化合物的pKa计算。而从G4MP2接收到的结果则有很大的偏差。计算出的pKa值对在计算过程中使用的比例因子alpha高度敏感。还计算了研究系统的理论和实验上可用的pKa值之间的均方根偏差(RMSD)。为了获得最佳结果,RMSD值低于0.8,这表明本文中提出的理论模型有望用于不同类别化合物的pKa计算。而从G4MP2接收到的结果则有很大的偏差。计算出的pKa值对在计算过程中使用的比例因子alpha高度敏感。还计算了研究系统的理论和实验上可用的pKa值之间的均方根偏差(RMSD)。为了获得最佳结果,RMSD值低于0.8,这表明本文中提出的理论模型有望用于不同类别化合物的pKa计算。
更新日期:2020-01-08
中文翻译:

计算水中和乙腈中选定的吡啶及其氮氧化物的pKa值。
吡啶,其N氧化物及其衍生物是令人兴奋的有机碱。这些化合物具有广泛的生物活性,通常用于合成。在这项工作中,基于水和乙腈中的热力学循环,完成了对酸解离常数(如吡啶的pKa,其N-氧化物及其衍生物)的理论计算结果。此外,还针对所研究的系统计算了气相碱度(GB)和质子亲和力(PA)值。所有的pKa值都是在气相中使用B3LYP,M06-2X和G4MP2方法获得的,将它们与PCM模型计算(在Hartree-Fock方法中)相结合,并使用四个不同的比例因子alpha。然后将理论GB,PA和pKa值与可用的实验值进行比较。从B3LYP和M06-2X方法获得的结果在质量上非常相似,并且与实验结果兼容,相关系数值R2高于0.9,而从G4MP2接收的结果则有很大差异。计算出的pKa值对计算过程中使用的比例因子alpha高度敏感。还计算了所研究系统的理论和实验上可用的pKa值之间的均方根偏差(RMSD)。为了获得最佳结果,RMSD值低于0.8,这表明这项工作中提出的理论模型有望用于不同类别化合物的pKa计算。而从G4MP2接收到的结果则有很大的偏差。计算出的pKa值对在计算过程中使用的比例因子alpha高度敏感。还计算了研究系统的理论和实验上可用的pKa值之间的均方根偏差(RMSD)。为了获得最佳结果,RMSD值低于0.8,这表明本文中提出的理论模型有望用于不同类别化合物的pKa计算。而从G4MP2接收到的结果则有很大的偏差。计算出的pKa值对在计算过程中使用的比例因子alpha高度敏感。还计算了研究系统的理论和实验上可用的pKa值之间的均方根偏差(RMSD)。为了获得最佳结果,RMSD值低于0.8,这表明本文中提出的理论模型有望用于不同类别化合物的pKa计算。




































 京公网安备 11010802027423号
京公网安备 11010802027423号