当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Abl-FBP17 mechanosensing system couples local plasma membrane curvature and stress fiber remodeling during mechanoadaptation.
Nature Communications ( IF 14.7 ) Pub Date : 2019-12-20 , DOI: 10.1038/s41467-019-13782-2 Asier Echarri 1 , Dácil M Pavón 1 , Sara Sánchez 1 , María García-García 1 , Enrique Calvo 2 , Carla Huerta-López 3 , Diana Velázquez-Carreras 3 , Christine Viaris de Lesegno 4 , Nicholas Ariotti 5 , Ana Lázaro-Carrillo 1, 6 , Raffaele Strippoli 7 , David De Sancho 8, 9 , Jorge Alegre-Cebollada 3 , Christophe Lamaze 4 , Robert G Parton 5, 10 , Miguel A Del Pozo 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-12-20 , DOI: 10.1038/s41467-019-13782-2 Asier Echarri 1 , Dácil M Pavón 1 , Sara Sánchez 1 , María García-García 1 , Enrique Calvo 2 , Carla Huerta-López 3 , Diana Velázquez-Carreras 3 , Christine Viaris de Lesegno 4 , Nicholas Ariotti 5 , Ana Lázaro-Carrillo 1, 6 , Raffaele Strippoli 7 , David De Sancho 8, 9 , Jorge Alegre-Cebollada 3 , Christophe Lamaze 4 , Robert G Parton 5, 10 , Miguel A Del Pozo 1
Affiliation
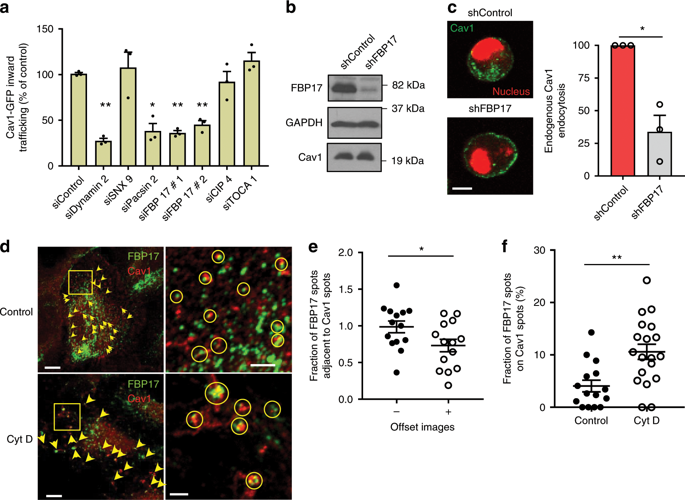
|
Cells remodel their structure in response to mechanical strain. However, how mechanical forces are translated into biochemical signals that coordinate the structural changes observed at the plasma membrane (PM) and the underlying cytoskeleton during mechanoadaptation is unclear. Here, we show that PM mechanoadaptation is controlled by a tension-sensing pathway composed of c-Abl tyrosine kinase and membrane curvature regulator FBP17. FBP17 is recruited to caveolae to induce the formation of caveolar rosettes. FBP17 deficient cells have reduced rosette density, lack PM tension buffering capacity under osmotic shock, and cannot adapt to mechanical strain. Mechanistically, tension is transduced to the FBP17 F-BAR domain by direct phosphorylation mediated by c-Abl, a mechanosensitive molecule. This modification inhibits FBP17 membrane bending activity and releases FBP17-controlled inhibition of mDia1-dependent stress fibers, favoring membrane adaptation to increased tension. This mechanoprotective mechanism adapts the cell to changes in mechanical tension by coupling PM and actin cytoskeleton remodeling.
中文翻译:

在机械适应过程中,Abl-FBP17机械传感系统将局部质膜曲率和应力纤维重塑耦合在一起。
细胞响应机械应变而重塑其结构。但是,尚不清楚如何将机械力转换为生物化学信号,以协调在机械适应过程中质膜(PM)和潜在的细胞骨架上观察到的结构变化。在这里,我们表明PM机械适应是由c-Abl酪氨酸激酶和膜曲率调节剂FBP17组成的张力传感途径控制的。FBP17被募集到小窝以诱导小窝玫瑰花结的形成。FBP17缺陷的细胞具有降低的玫瑰花结密度,渗透压下缺乏PM张力缓冲能力,并且不能适应机械应变。机械上,张力是由机械敏感分子c-Abl介导的直接磷酸化作用转导至FBP17 F-BAR结构域的。此修饰可抑制FBP17膜的弯曲活性,并释放FBP17控制的对mDia1依赖性应力纤维的抑制作用,有利于膜适应增加的张力。这种机械保护机制通过偶联PM和肌动蛋白细胞骨架重塑使细胞适应机械张力的变化。
更新日期:2019-12-20
中文翻译:

在机械适应过程中,Abl-FBP17机械传感系统将局部质膜曲率和应力纤维重塑耦合在一起。
细胞响应机械应变而重塑其结构。但是,尚不清楚如何将机械力转换为生物化学信号,以协调在机械适应过程中质膜(PM)和潜在的细胞骨架上观察到的结构变化。在这里,我们表明PM机械适应是由c-Abl酪氨酸激酶和膜曲率调节剂FBP17组成的张力传感途径控制的。FBP17被募集到小窝以诱导小窝玫瑰花结的形成。FBP17缺陷的细胞具有降低的玫瑰花结密度,渗透压下缺乏PM张力缓冲能力,并且不能适应机械应变。机械上,张力是由机械敏感分子c-Abl介导的直接磷酸化作用转导至FBP17 F-BAR结构域的。此修饰可抑制FBP17膜的弯曲活性,并释放FBP17控制的对mDia1依赖性应力纤维的抑制作用,有利于膜适应增加的张力。这种机械保护机制通过偶联PM和肌动蛋白细胞骨架重塑使细胞适应机械张力的变化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号