当前位置:
X-MOL 学术
›
Lancet Infect Dis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with Gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial.
The Lancet Infectious Diseases ( IF 36.4 ) Pub Date : 2019-12-19 , DOI: 10.1016/s1473-3099(19)30574-2 Michael S Niederman 1 , Jeff Alder 2 , Matteo Bassetti 3 , Francis Boateng 4 , Bin Cao 5 , Kevin Corkery 6 , Rajiv Dhand 7 , Keith S Kaye 8 , Robert Lawatscheck 9 , Patrick McLeroth 10 , David P Nicolau 11 , Chen Wang 5 , G Christopher Wood 12 , Richard G Wunderink 13 , Jean Chastre 14
The Lancet Infectious Diseases ( IF 36.4 ) Pub Date : 2019-12-19 , DOI: 10.1016/s1473-3099(19)30574-2 Michael S Niederman 1 , Jeff Alder 2 , Matteo Bassetti 3 , Francis Boateng 4 , Bin Cao 5 , Kevin Corkery 6 , Rajiv Dhand 7 , Keith S Kaye 8 , Robert Lawatscheck 9 , Patrick McLeroth 10 , David P Nicolau 11 , Chen Wang 5 , G Christopher Wood 12 , Richard G Wunderink 13 , Jean Chastre 14
Affiliation
|
BACKGROUND
Treatment of ventilated pneumonia is often unsuccessful, even when patients are treated according to established guidelines. Therefore, we aimed to investigate the efficacy of the combination drug device Amikacin Inhale as an adjunctive therapy to intravenous standard-of-care antibiotics for pneumonia caused by Gram-negative pathogens in intubated and mechanically ventilated patients.
METHODS
INHALE was a prospective, double-blind, randomised, placebo-controlled, phase 3 study comprising two trials (INHALE 1 and INHALE 2) done in 153 hospital intensive-care units in 25 countries. Eligible patients were aged 18 years or older; had pneumonia that had been diagnosed by chest radiography and that was documented as being caused by or showing two risk factors for a Gram-negative, multidrug-resistant pathogen; were intubated and mechanically ventilated; had impaired oxygenation within 48 h before screening; and had a modified Clinical Pulmonary Infection Score of at least 6. Patients were stratified by region and disease severity (according to their Acute Physiology and Chronic Health Evaluation [APACHE] II score) and randomly assigned (1:1) via an interactive voice-recognition system to receive 400 mg amikacin (Amikacin Inhale) or saline placebo, both of which were aerosolised, administered every 12 h for 10 days via the same synchronised inhalation system, and given alongside standard-of-care intravenous antibiotics. All patients and all staff involved in administering devices and monitoring outcomes were masked to treatment assignment. The primary endpoint, survival at days 28-32, was analysed in all patients who received at least one dose of study drug, were infected with a Gram-negative pathogen, and had an APACHE II score of at least 10 at diagnosis. Safety analyses were done in all patients who received at least one dose of study drug. This study is registered with ClinicalTrials.gov, numbers NCT01799993 and NCT00805168.
FINDINGS
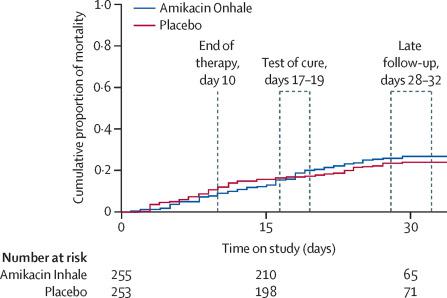
Between April 13, 2013, and April 7, 2017, 807 patients were assessed for eligibility and 725 were randomly assigned to Amikacin Inhale (362 patients) or aerosolised placebo (363 patients). 712 patients received at least one dose of study drug (354 in the Amikacin Inhale group and 358 in the placebo group), although one patient assigned to Amikacin Inhale received placebo in error and was included in the placebo group for safety analyses. 508 patients (255 in the Amikacin Inhale group and 253 in the placebo group) were assessed for the primary endpoint. We found no between-group difference in survival: 191 (75%) patients in the Amikacin Inhale group versus 196 (77%) patients in the placebo group survived until days 28-32 (odds ratio 0·841, 95% CI 0·554-1·277; p=0·43). Similar proportions of patients in the two treatment groups had a treatment-emergent adverse event (295 [84%] of 353 patients in the Amikacin Inhale group vs 303 [84%] of 359 patients in the placebo group) or a serious treatment-emergent adverse event (101 [29%] patients vs 97 [27%] patients).
INTERPRETATION
Our findings do not support use of inhaled amikacin adjunctive to standard-of-care intravenous therapy in mechanically ventilated patients with Gram-negative pneumonia.
FUNDING
Bayer AG.
中文翻译:

机械通气革兰氏阴性肺炎 (INHALE) 患者吸入阿米卡星辅助静脉内标准护理抗生素:一项双盲、随机、安慰剂对照、3 期优势试验。
背景技术通气性肺炎的治疗常常是不成功的,即使当患者根据既定指南进行治疗时也是如此。因此,我们旨在研究联合药物装置阿米卡星吸入作为插管和机械通气患者革兰氏阴性病原体引起的肺炎静脉内标准护理抗生素的辅助治疗的疗效。方法 INHALE 是一项前瞻性、双盲、随机、安慰剂对照的 3 期研究,包括在 25 个国家的 153 个医院重症监护病房进行的两项试验(INHALE 1 和 INHALE 2)。符合条件的患者年龄在 18 岁或以上;患有通过胸片诊断的肺炎,并且被记录为由革兰氏阴性、多重耐药病原体引起或显示出两个危险因素;插管和机械通气;筛选前 48 小时内氧合作用受损;患者的临床肺部感染评分至少为 6识别系统接受 400 mg 阿米卡星 (Amikacin Inhale) 或生理盐水安慰剂,两者均被雾化,通过相同的同步吸入系统每 12 小时给药一次,持续 10 天,并与标准护理静脉注射抗生素一起给药。所有患者和所有参与设备管理和监测结果的工作人员都对治疗分配设盲。主要终点,第 28-32 天的存活率,在接受至少一剂研究药物、感染革兰氏阴性病原体且诊断时 APACHE II 评分至少为 10 的所有患者中进行了分析。对接受至少一剂研究药物的所有患者进行安全性分析。本研究已在 ClinicalTrials.gov 注册,编号为 NCT01799993 和 NCT00805168。2013 年 4 月 13 日至 2017 年 4 月 7 日期间,对 807 名患者进行了资格评估,其中 725 名患者被随机分配到阿米卡星吸入组(362 名患者)或雾化安慰剂组(363 名患者)。712 名患者接受了至少一剂研究药物(阿米卡星吸入组 354 名,安慰剂组 358 名),尽管分配到阿米卡星吸入组的一名患者错误地接受了安慰剂,并被纳入安慰剂组进行安全性分析。评估了 508 名患者(阿米卡星吸入组 255 名和安慰剂组 253 名)的主要终点。我们发现存活率没有组间差异:阿米卡星吸入组 191 名 (75%) 患者与安慰剂组 196 名 (77%) 患者存活至第 28-32 天(比值比 0·841,95% CI 0· 554-1·277;p=0·43)。两个治疗组中有相似比例的患者出现治疗中出现的不良事件(阿米卡星吸入组 353 名患者中的 295 名 [84%] vs 安慰剂组 359 名患者中的 303 名 [84%])或严重的治疗中出现的不良事件不良事件(101 [29%] 患者 vs 97 [27%] 患者)。解释 我们的研究结果不支持在机械通气革兰氏阴性肺炎患者中使用吸入阿米卡星辅助标准护理静脉治疗。资金拜耳公司。
更新日期:2020-02-27
中文翻译:

机械通气革兰氏阴性肺炎 (INHALE) 患者吸入阿米卡星辅助静脉内标准护理抗生素:一项双盲、随机、安慰剂对照、3 期优势试验。
背景技术通气性肺炎的治疗常常是不成功的,即使当患者根据既定指南进行治疗时也是如此。因此,我们旨在研究联合药物装置阿米卡星吸入作为插管和机械通气患者革兰氏阴性病原体引起的肺炎静脉内标准护理抗生素的辅助治疗的疗效。方法 INHALE 是一项前瞻性、双盲、随机、安慰剂对照的 3 期研究,包括在 25 个国家的 153 个医院重症监护病房进行的两项试验(INHALE 1 和 INHALE 2)。符合条件的患者年龄在 18 岁或以上;患有通过胸片诊断的肺炎,并且被记录为由革兰氏阴性、多重耐药病原体引起或显示出两个危险因素;插管和机械通气;筛选前 48 小时内氧合作用受损;患者的临床肺部感染评分至少为 6识别系统接受 400 mg 阿米卡星 (Amikacin Inhale) 或生理盐水安慰剂,两者均被雾化,通过相同的同步吸入系统每 12 小时给药一次,持续 10 天,并与标准护理静脉注射抗生素一起给药。所有患者和所有参与设备管理和监测结果的工作人员都对治疗分配设盲。主要终点,第 28-32 天的存活率,在接受至少一剂研究药物、感染革兰氏阴性病原体且诊断时 APACHE II 评分至少为 10 的所有患者中进行了分析。对接受至少一剂研究药物的所有患者进行安全性分析。本研究已在 ClinicalTrials.gov 注册,编号为 NCT01799993 和 NCT00805168。2013 年 4 月 13 日至 2017 年 4 月 7 日期间,对 807 名患者进行了资格评估,其中 725 名患者被随机分配到阿米卡星吸入组(362 名患者)或雾化安慰剂组(363 名患者)。712 名患者接受了至少一剂研究药物(阿米卡星吸入组 354 名,安慰剂组 358 名),尽管分配到阿米卡星吸入组的一名患者错误地接受了安慰剂,并被纳入安慰剂组进行安全性分析。评估了 508 名患者(阿米卡星吸入组 255 名和安慰剂组 253 名)的主要终点。我们发现存活率没有组间差异:阿米卡星吸入组 191 名 (75%) 患者与安慰剂组 196 名 (77%) 患者存活至第 28-32 天(比值比 0·841,95% CI 0· 554-1·277;p=0·43)。两个治疗组中有相似比例的患者出现治疗中出现的不良事件(阿米卡星吸入组 353 名患者中的 295 名 [84%] vs 安慰剂组 359 名患者中的 303 名 [84%])或严重的治疗中出现的不良事件不良事件(101 [29%] 患者 vs 97 [27%] 患者)。解释 我们的研究结果不支持在机械通气革兰氏阴性肺炎患者中使用吸入阿米卡星辅助标准护理静脉治疗。资金拜耳公司。































 京公网安备 11010802027423号
京公网安备 11010802027423号