当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scandium(III) Triflate Catalyzed Direct Synthesis of N-Unprotected Ketimines.
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.orglett.9b04038 Yuta Kondo 1 , Tetsuya Kadota 1 , Yoshinobu Hirazawa 1 , Kazuhiro Morisaki 1 , Hiroyuki Morimoto 1 , Takashi Ohshima 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.orglett.9b04038 Yuta Kondo 1 , Tetsuya Kadota 1 , Yoshinobu Hirazawa 1 , Kazuhiro Morisaki 1 , Hiroyuki Morimoto 1 , Takashi Ohshima 1
Affiliation
|
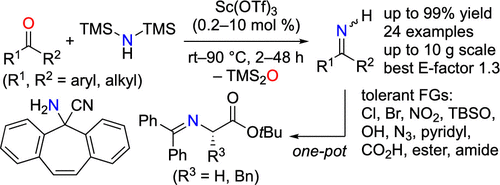
N-Unprotected ketimines are useful substrates and intermediates for synthesizing valuable nitrogen-containing compounds, but their potential applicability is limited by the available synthetic methods. To address this issue, we report a scandium(III) triflate catalyzed direct synthesis of N-unprotected ketimines. Using commercially available reagents and Lewis acid catalysts, ketones were directly transformed into the corresponding N-unprotected ketimines in high yields with broad functional group tolerance, even in multigram scales. The reactions were readily applicable for one-pot synthesis of important compounds such as a glycine Schiff base without isolation of N-unprotected ketimine intermediates. Preliminary mechanistic studies to clarify the reaction mechanism are also described.
中文翻译:

三氟甲磺酸(III)催化N-未保护的酮亚胺的直接合成。
N-未保护的酮亚胺是用于合成有价值的含氮化合物的有用底物和中间体,但其潜在的适用性受到可用合成方法的限制。为了解决这个问题,我们报告了三氟甲磺酸((III)催化的N-未保护酮亚胺的直接合成。使用可商购的试剂和路易斯酸催化剂,将酮以高收率直接转化为相应的N-未保护的酮亚胺,具有宽泛的官能团耐受性,甚至以毫克计。该反应易于用于重要化合物(例如甘氨酸席夫碱)的一锅合成,而无需分离N-未保护的酮亚胺中间体。还介绍了初步的机理研究以阐明反应机理。
更新日期:2019-12-19
中文翻译:

三氟甲磺酸(III)催化N-未保护的酮亚胺的直接合成。
N-未保护的酮亚胺是用于合成有价值的含氮化合物的有用底物和中间体,但其潜在的适用性受到可用合成方法的限制。为了解决这个问题,我们报告了三氟甲磺酸((III)催化的N-未保护酮亚胺的直接合成。使用可商购的试剂和路易斯酸催化剂,将酮以高收率直接转化为相应的N-未保护的酮亚胺,具有宽泛的官能团耐受性,甚至以毫克计。该反应易于用于重要化合物(例如甘氨酸席夫碱)的一锅合成,而无需分离N-未保护的酮亚胺中间体。还介绍了初步的机理研究以阐明反应机理。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号