当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Electrocatalytic Water Oxidation to Produce H2O2 Using a C,N Codoped TiO2 Electrode in an Acidic Electrolyte.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-12-18 , DOI: 10.1021/acsami.9b16937
Sheng-guo Xue , Lu Tang , Yi-kun Tang , Chu-xuan Li , Meng-li Li , Jing-ju Zhou , Wei Chen , Feng Zhu , Jun Jiang
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-12-18 , DOI: 10.1021/acsami.9b16937
Sheng-guo Xue , Lu Tang , Yi-kun Tang , Chu-xuan Li , Meng-li Li , Jing-ju Zhou , Wei Chen , Feng Zhu , Jun Jiang
|
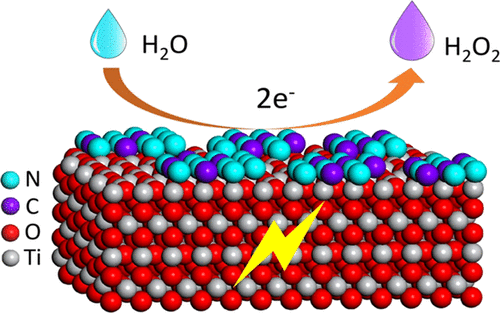
Production of hydrogen peroxide (H2O2) via in situ electrochemical water oxidation possesses great potential applications in the energy and environment fields. In this work, for the first time, we reported a C,N codoped TiO2 electrode for selective electrocatalytic water oxidation to produce H2O2 in an acidic electrolyte. An electrochemical anodic oxidation method combined with postcalcination in the presence of urea was applied to fabricate such a C,N codoped TiO2 electrode, which was evidenced by detail structural characterizations. The calcination temperature and urea atmosphere were found to play key roles in its catalytic performances; the optimized 600N sample exhibited an onset potential of 2.66 V (vs Ag/AgCl) and a Tafel slope of 51 mV dec-1 at pH 3. Under the optimal applied potential, the cumulative H2O2 concentration for this sample reached 0.29 μmol L-1 cm-2 h-1. More importantly, a simple recalcination strategy was developed to recover the deactivation electrode. This study proposed an efficient C,N codoped TiO2 electrode toward water oxidation to selectively produce H2O2 in the acidic electrolyte, which could be further used to in situ generate H2O2 for the energy- and environment-related fields with water as the precursor.
中文翻译:

在酸性电解质中使用C,N共掺杂的TiO2电极进行选择性电催化水氧化生成H2O2。
通过原位电化学水氧化生产过氧化氢(H2O2)在能源和环境领域具有巨大的潜在应用。在这项工作中,我们首次报道了一种碳,氮共掺杂的TiO2电极,用于选择性电催化水氧化以在酸性电解质中生成H2O2。电化学阳极氧化方法与尿素存在下的后煅烧相结合,被用于制造这种C,N共掺杂的TiO2电极,这通过详细的结构表征得到了证明。发现煅烧温度和尿素气氛对其催化性能起关键作用。经过优化的600N样品在pH 3下的起始电势为2.66 V(vs Ag / AgCl),Tafel斜率为51 mV dec-1。该样品的累积H2O2浓度达到0.29μmolL-1 cm-2 h-1。更重要的是,开发了一种简单的重新煅烧策略来恢复失活电极。这项研究提出了一种高效的C,N共掺杂的TiO2电极,用于水氧化以在酸性电解质中选择性地产生H2O2,该电极可以进一步用于以水为前体原位产生H2O2,用于与能源和环境相关的领域。
更新日期:2020-01-14
中文翻译:

在酸性电解质中使用C,N共掺杂的TiO2电极进行选择性电催化水氧化生成H2O2。
通过原位电化学水氧化生产过氧化氢(H2O2)在能源和环境领域具有巨大的潜在应用。在这项工作中,我们首次报道了一种碳,氮共掺杂的TiO2电极,用于选择性电催化水氧化以在酸性电解质中生成H2O2。电化学阳极氧化方法与尿素存在下的后煅烧相结合,被用于制造这种C,N共掺杂的TiO2电极,这通过详细的结构表征得到了证明。发现煅烧温度和尿素气氛对其催化性能起关键作用。经过优化的600N样品在pH 3下的起始电势为2.66 V(vs Ag / AgCl),Tafel斜率为51 mV dec-1。该样品的累积H2O2浓度达到0.29μmolL-1 cm-2 h-1。更重要的是,开发了一种简单的重新煅烧策略来恢复失活电极。这项研究提出了一种高效的C,N共掺杂的TiO2电极,用于水氧化以在酸性电解质中选择性地产生H2O2,该电极可以进一步用于以水为前体原位产生H2O2,用于与能源和环境相关的领域。







































 京公网安备 11010802027423号
京公网安备 11010802027423号