当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-natural Cofactor and Formate-Driven Reductive Carboxylation of Pyruvate.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-01-21 , DOI: 10.1002/anie.201915303 Xiaojia Guo 1, 2 , Yuxue Liu 1 , Qian Wang 1, 3 , Xueying Wang 1, 3 , Qing Li 1, 2 , Wujun Liu 1 , Zongbao K Zhao 1, 3, 4
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-01-21 , DOI: 10.1002/anie.201915303 Xiaojia Guo 1, 2 , Yuxue Liu 1 , Qian Wang 1, 3 , Xueying Wang 1, 3 , Qing Li 1, 2 , Wujun Liu 1 , Zongbao K Zhao 1, 3, 4
Affiliation
|
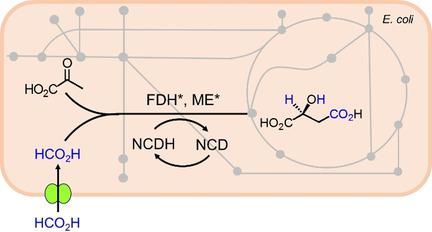
A non-natural cofactor and formate driven system for reductive carboxylation of pyruvate is presented. A formate dehydrogenase (FDH) mutant, FDH*, that favors a non-natural redox cofactor, nicotinamide cytosine dinucleotide (NCD), for generation of a dedicated reducing equivalent at the expense of formate were acquired. By coupling FDH* and NCD-dependent malic enzyme (ME*), the successful utilization of formate is demonstrated as both CO2 source and electron donor for reductive carboxylation of pyruvate with a perfect stoichiometry between formate and malate. When 13 C-isotope-labeled formate was used in in vitro trials, up to 53 % of malate had labeled carbon atom. Upon expression of FDH* and ME* in the model host E. coli, the engineered strain produced more malate in the presence of formate and NCD. This work provides an alternative and atom-economic strategy for CO2 fixation where formate is used in lieu of CO2 and offers dedicated reducing power.
中文翻译:

丙酮酸的非天然辅因子和甲酸酯驱动的还原性羧化反应。
提出了一种用于丙酮酸还原羧化的非天然辅因子和甲酸盐驱动的系统。获得了有利于非天然氧化还原辅因子烟酰胺胞嘧啶二核苷酸(NCD)的甲酸盐脱氢酶(FDH)突变体FDH *,以牺牲甲酸盐为代价生成了专用的还原当量。通过偶联FDH *和NCD依赖性苹果酸酶(ME *),成功地利用了甲酸盐作为CO2源和电子供体,用于丙酮酸盐和甲酸盐之间具有理想化学计量的丙酮酸还原羧化反应。当在体外试验中使用13 C同位素标记的甲酸酯时,多达53%的苹果酸具有标记的碳原子。在模型宿主大肠杆菌中表达FDH *和ME *后,经过改造的菌株在甲酸和NCD的存在下产生更多的苹果酸。
更新日期:2020-01-22
中文翻译:

丙酮酸的非天然辅因子和甲酸酯驱动的还原性羧化反应。
提出了一种用于丙酮酸还原羧化的非天然辅因子和甲酸盐驱动的系统。获得了有利于非天然氧化还原辅因子烟酰胺胞嘧啶二核苷酸(NCD)的甲酸盐脱氢酶(FDH)突变体FDH *,以牺牲甲酸盐为代价生成了专用的还原当量。通过偶联FDH *和NCD依赖性苹果酸酶(ME *),成功地利用了甲酸盐作为CO2源和电子供体,用于丙酮酸盐和甲酸盐之间具有理想化学计量的丙酮酸还原羧化反应。当在体外试验中使用13 C同位素标记的甲酸酯时,多达53%的苹果酸具有标记的碳原子。在模型宿主大肠杆菌中表达FDH *和ME *后,经过改造的菌株在甲酸和NCD的存在下产生更多的苹果酸。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号