当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Refolding Dynamics of gp41 from Pre-fusion to Pre-hairpin States during HIV-1 Entry.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-12-31 , DOI: 10.1021/acs.jcim.9b00746
Mengna Lin 1 , Lin-Tai Da 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-12-31 , DOI: 10.1021/acs.jcim.9b00746
Mengna Lin 1 , Lin-Tai Da 1
Affiliation
|
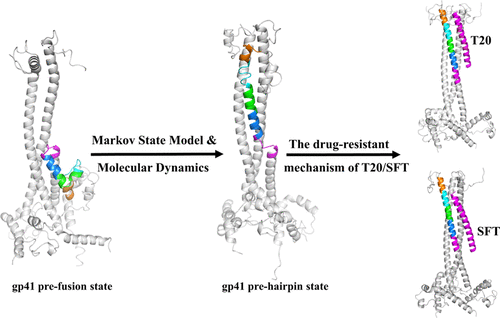
The HIV-1 infection is triggered by the binding of the viral envelope glycoprotein (Env) gp120-gp41 trimer to host-cell receptor CD4 and co-receptor CCR5/CXCR4, which leads to substantial conformational changes of Env, that is, structural transition of gp120 from a closed to an open state followed by gp41 refolding from pre-fusion to post-fusion states. The latter finally promotes membrane fusion, likely via visiting a critical pre-hairpin state of gp41. The complete conformational dynamics of the pre-hairpin formation at atomic resolution, however, is still unknown. Here, by constructing a Markov state model based on the all-atom molecular dynamics (MD) with an aggregated simulation time of ∼24 μs, we reveal the gp41 refolding dynamics from pre-fusion to pre-hairpin state and the key metastable states involved. Moreover, we further explored the drug resistance mechanism of two C-terminal heptad repeat-derived gp41 inhibitors, T20 and sifuvirtide, based on the constructed inhibitor-bound gp41 pre-hairpin complexes. The results indicate that these two inhibitors have distinct binding sites on gp41 but share a common drug resistance region that usually exhibits a helical structure in the pre-hairpin state yet adopts various secondary structures in other metastable states. Moreover, we conducted several mutant MD simulations to further investigate the mechanisms of how some drug-resistant mutations might affect the pre-hairpin formation, which in turn prevent the inhibitor recognition. Our findings provide deep structural insights into the molecular mechanisms of the pre-hairpin formation for gp41, which helps to guide future anti-HIV drug design.
中文翻译:

HIV-1进入过程中gp41从融合前到发夹前状态的重折叠动力学。
HIV-1感染是由病毒包膜糖蛋白(Env)gp120-gp41三聚体与宿主细胞受体CD4和共受体CCR5 / CXCR4的结合触发的,这导致Env的构象发生实质性变化,即结构转变gp120从闭合状态转变为打开状态,然后gp41从融合前状态重新融合到融合后状态。后者最终可能通过访问gp41的关键发夹前状态来促进膜融合。然而,在原子分辨率下前发夹结构的完整构象动力学仍然是未知的。在此,通过基于全原子分子动力学(MD)且模拟时间约为24μs的马尔可夫状态模型构建,我们揭示了gp41从融合前到发夹前状态的重折叠动力学以及涉及的关键亚稳态。而且,我们基于构建的与抑制剂结合的gp41前发夹复合物,进一步探讨了两种C端七肽重复序列衍生的gp41抑制剂T20和sifuvirtide的耐药机制。结果表明,这两种抑制剂在gp41上具有不同的结合位点,但共有一个共同的耐药区域,该区域通常在发夹前状态下呈螺旋结构,而在其他亚稳态下则具有多种二级结构。此外,我们进行了几次突变体MD模拟,以进一步研究某些耐药性突变如何影响发夹前形成的机制,从而阻止了抑制剂的识别。我们的发现为gp41的发夹前形成的分子机制提供了深刻的结构见解,有助于指导未来的抗HIV药物设计。
更新日期:2019-12-31
中文翻译:

HIV-1进入过程中gp41从融合前到发夹前状态的重折叠动力学。
HIV-1感染是由病毒包膜糖蛋白(Env)gp120-gp41三聚体与宿主细胞受体CD4和共受体CCR5 / CXCR4的结合触发的,这导致Env的构象发生实质性变化,即结构转变gp120从闭合状态转变为打开状态,然后gp41从融合前状态重新融合到融合后状态。后者最终可能通过访问gp41的关键发夹前状态来促进膜融合。然而,在原子分辨率下前发夹结构的完整构象动力学仍然是未知的。在此,通过基于全原子分子动力学(MD)且模拟时间约为24μs的马尔可夫状态模型构建,我们揭示了gp41从融合前到发夹前状态的重折叠动力学以及涉及的关键亚稳态。而且,我们基于构建的与抑制剂结合的gp41前发夹复合物,进一步探讨了两种C端七肽重复序列衍生的gp41抑制剂T20和sifuvirtide的耐药机制。结果表明,这两种抑制剂在gp41上具有不同的结合位点,但共有一个共同的耐药区域,该区域通常在发夹前状态下呈螺旋结构,而在其他亚稳态下则具有多种二级结构。此外,我们进行了几次突变体MD模拟,以进一步研究某些耐药性突变如何影响发夹前形成的机制,从而阻止了抑制剂的识别。我们的发现为gp41的发夹前形成的分子机制提供了深刻的结构见解,有助于指导未来的抗HIV药物设计。

































 京公网安备 11010802027423号
京公网安备 11010802027423号