当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2‐Alkynyl‐ and2‐Amino‐12H‐benzothiazolo[2,3‐b]quinazolin‐12‐ones and Their Inhibitory Potential against Monoamine Oxidase A and B
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-12-17 , DOI: 10.1002/slct.201903300 Behzad Jafari 1 , Saquib Jalil 2 , Sumera Zaib 2 , Sayfidin Safarov 1, 3 , Muattar Khalikova 1, 3 , Djurabay Khalikov 1, 3 , Meirambek Ospanov 1, 4 , Nazym Yelibayeva 1, 4 , Shynar Zhumagalieva 4 , Zharylkasyn A. Abilov 4 , Mirgul Z. Turmukhanova 4 , Sergey N. Kalugin 4 , Ghazwan Ali Salman 1, 5 , Peter Ehlers 1 , Abdul Hameed 2 , Jamshed Iqbal 2 , Peter Langer 1, 6
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-12-17 , DOI: 10.1002/slct.201903300 Behzad Jafari 1 , Saquib Jalil 2 , Sumera Zaib 2 , Sayfidin Safarov 1, 3 , Muattar Khalikova 1, 3 , Djurabay Khalikov 1, 3 , Meirambek Ospanov 1, 4 , Nazym Yelibayeva 1, 4 , Shynar Zhumagalieva 4 , Zharylkasyn A. Abilov 4 , Mirgul Z. Turmukhanova 4 , Sergey N. Kalugin 4 , Ghazwan Ali Salman 1, 5 , Peter Ehlers 1 , Abdul Hameed 2 , Jamshed Iqbal 2 , Peter Langer 1, 6
Affiliation
|
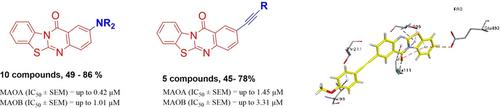
2‐Alkynyl‐ and 2‐aminobenzothiazolo[2,3‐b]quinazolin‐12‐ones have been synthesized by Palladium catalysed Buchwald‐Hartwig and Sonogashira reactions. Synthesized derivatives were further evaluated for their role as potential inhibitors of monoamine oxidase A and B (MAO−A and B) isozymes. Most compounds possess moderate to excellent inhibitory potential against MAO−A and MAO−B. The 2‐amino‐substituted derivatives show a significantly higher activity as compared to 2‐alkynyl‐ and previously reported 2‐aryl derivatives. Studied compounds might be employed as novel monoamine oxidase inhibitors and may provide insights for the development of new drug candidates against neurological diseases.
中文翻译:

2-炔基和2-氨基-12H-苯并噻唑并[2,3-b]喹唑啉-12-酮的合成及其对单胺氧化酶A和B的抑制潜力
钯催化的Buchwald-Hartwig和Sonogashira反应合成了2-炔基和2-氨基苯并噻唑[2,3- b ]喹唑啉-12-。进一步评估了合成衍生物作为单胺氧化酶A和B(MAO-A和B)同工酶的潜在抑制剂的作用。大多数化合物具有对MAO-A和MAO-B的中度到极好的抑制潜能。与2-炔基和以前报道的2-芳基衍生物相比,2-氨基取代的衍生物显示出明显更高的活性。研究的化合物可以用作新型单胺氧化酶抑制剂,并且可以为开发抗神经系统疾病的新药物提供见识。
更新日期:2019-12-18
中文翻译:

2-炔基和2-氨基-12H-苯并噻唑并[2,3-b]喹唑啉-12-酮的合成及其对单胺氧化酶A和B的抑制潜力
钯催化的Buchwald-Hartwig和Sonogashira反应合成了2-炔基和2-氨基苯并噻唑[2,3- b ]喹唑啉-12-。进一步评估了合成衍生物作为单胺氧化酶A和B(MAO-A和B)同工酶的潜在抑制剂的作用。大多数化合物具有对MAO-A和MAO-B的中度到极好的抑制潜能。与2-炔基和以前报道的2-芳基衍生物相比,2-氨基取代的衍生物显示出明显更高的活性。研究的化合物可以用作新型单胺氧化酶抑制剂,并且可以为开发抗神经系统疾病的新药物提供见识。































 京公网安备 11010802027423号
京公网安备 11010802027423号