Polymer ( IF 4.1 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.polymer.2018.03.024 Yang Tang , Song-Qing Zhao , Na Liu , Zong-Quan Wu
|
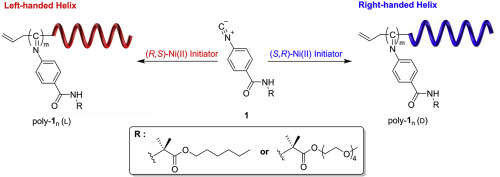
Living polymerization of achiral phenyl isocyanide using a simply prepared chiral π-allylnickel complex as initiator was found to proceed in helix-sense-selective manner. The polymerization of achiral phenyl isocyanide, 4-isocyanobenzoyl-2-aminoisobutyric acid hexyl ester (1a) produced optically active helical poly-1am (L or D) whose chirality totally come from the helical conformation without containing any other chiral atoms. The monomer-structural effect on the helix-sense-selectivity was examined and indicated the long alkyl group in the monomer is not essential for the helix-sense-selective polymerization. The effect of chiral phosphine ligand was also investigated. The success of the helix-sense-selective polymerizations indicated the high activity of π-allylnickel complexes. Finally, polymerization of 2,5-dibromo-3-hexylthiophene (3HT) and subsequent addition of 1a in the presence of chiral π-allylnickel complex as a single catalyst afforded P3HTm-b-poly-1an with nonhelical P3HT and helical poly(phenyl isocyanide) (PPI) segments, which may be used as unique chiral organic materials in the future.
中文翻译:

-有规立构的螺旋聚(苯基异氰化物)和聚(3-己基噻吩)的简便合成嵌段共聚物使用手性的πallylnickel络合物作为引发剂-聚(苯基异氰化物)
发现使用简单制备的手性π-烯丙基镍配合物作为引发剂的非手性苯基异氰酸酯的活性聚合以螺旋-感觉-选择性方式进行。非手性苯基异氰酸酯,4-异氰基苯甲酰基-2-氨基异丁酸己酯(1a)的聚合反应生成了旋光螺旋聚1a m(L或D),其手性完全来自螺旋构象,不含任何其他手性原子。考察了单体结构对螺旋-感官选择性的影响,并表明单体中的长烷基对于螺旋-感官选择性聚合不是必需的。还研究了手性膦配体的作用。螺旋-感觉-选择性聚合的成功表明了π-烯丙基镍配合物的高活性。最后,将2,5-二溴-3-己基噻吩(3HT)聚合,然后在手性π-烯丙基镍配合物作为单一催化剂的情况下添加1a,得到P3HT m - b -poly- 1a n 具有非螺旋P3HT和螺旋聚(异氰酸苯酯)(PPI)链段,将来可能用作独特的手性有机材料。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号