当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of new organic superacids with fused and isolated pyrrole and cyclopentadiene rings and assessment of effect of –BX2 (X = H, F, Cl, CN) substituents on the acidity enhancement: A DFT analysis
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-12-16 , DOI: 10.1002/qua.26144 Hasibeh Parsazadeh 1 , Younes Valadbeigi 2 , Morteza Rouhani 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-12-16 , DOI: 10.1002/qua.26144 Hasibeh Parsazadeh 1 , Younes Valadbeigi 2 , Morteza Rouhani 1
Affiliation
|
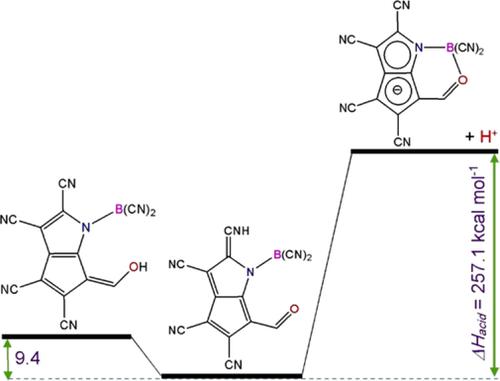
New classes of organic Brønsted acids were designed with pyrrole and cyclopentadiene scaffolds, and their acidity was assessed theoretically by the B3LYP/6‐311++G(d,p) method. The hydrogen atom of NH group in pyrrole was substituted by an –BX2 (X = H, F, Cl, CN, CF3). The boron atom stabilizes the conjugated bases by interaction with the center of negative charge after deprotonation. The acidity of the compounds was promoted by substitution of the hydrogen atoms of the rings with CN moiety as a strong electron withdrawing group. Also, after deprotonation, delocalization of the negative charge in both pyrrole and cyclopentadiene rings causes stability of the conjugated bases and consequently enhances the acidity. The charge delocalization in the neutral acids and their conjugated bases was compared using nucleus‐independent chemical shift index. Enthalpies and Gibbs free energies of deprotonation in gas phase, ∆Hacid and ∆Gacid, were used as a measure of acidity. Both compounds with isolated and fused pyrrole and cyclopentadiene rings were investigated and it was found that the formers are more acidic. Using these strategies, several acids and superacids with wide range of acidity with ∆Gacid values of 244 to 328 kcal mol−1 were obtained.
中文翻译:

具有稠合和分离的吡咯和环戊二烯环的新型有机超强酸的设计以及–BX2(X = H,F,Cl,CN)取代基对酸度增强的影响评估:DFT分析
用吡咯和环戊二烯支架设计了新的有机布朗斯台德酸类,并通过B3LYP / 6-311 ++ G(d,p)方法从理论上评估了其酸度。吡咯中NH基团的氢原子被–BX 2(X = H,F,Cl,CN,CF 3取代))。硼原子在去质子化后通过与负电荷中心相互作用来稳定共轭碱基。通过用CN部分作为强吸电子基团取代环的氢原子,可以提高化合物的酸度。而且,在去质子化之后,吡咯和环戊二烯环中的负电荷的离域化引起共轭碱的稳定性,因此增强了酸度。使用不依赖核的化学位移指数比较了中性酸及其共轭碱中的电荷离域。气相中去质子的焓和吉布斯自由能,∆ H酸和∆ G酸用作酸度的量度。研究了具有分离的和稠合的吡咯和环戊二烯环的两种化合物,发现前者的酸性更高。使用这些策略,获得了多种酸和宽酸度的ΔG酸值为244至328 kcal mol -1的酸。
更新日期:2020-03-02
中文翻译:

具有稠合和分离的吡咯和环戊二烯环的新型有机超强酸的设计以及–BX2(X = H,F,Cl,CN)取代基对酸度增强的影响评估:DFT分析
用吡咯和环戊二烯支架设计了新的有机布朗斯台德酸类,并通过B3LYP / 6-311 ++ G(d,p)方法从理论上评估了其酸度。吡咯中NH基团的氢原子被–BX 2(X = H,F,Cl,CN,CF 3取代))。硼原子在去质子化后通过与负电荷中心相互作用来稳定共轭碱基。通过用CN部分作为强吸电子基团取代环的氢原子,可以提高化合物的酸度。而且,在去质子化之后,吡咯和环戊二烯环中的负电荷的离域化引起共轭碱的稳定性,因此增强了酸度。使用不依赖核的化学位移指数比较了中性酸及其共轭碱中的电荷离域。气相中去质子的焓和吉布斯自由能,∆ H酸和∆ G酸用作酸度的量度。研究了具有分离的和稠合的吡咯和环戊二烯环的两种化合物,发现前者的酸性更高。使用这些策略,获得了多种酸和宽酸度的ΔG酸值为244至328 kcal mol -1的酸。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号