当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
External Electric Field—Phenyl interaction boosts hydrosilylation of substituted alkynes to α‐vinylsilane
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-12-14 , DOI: 10.1002/qua.26134 Ming‐Xia Zhang 1 , Hong‐Liang Xu 1 , Zhong‐Min Su 1, 2
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-12-14 , DOI: 10.1002/qua.26134 Ming‐Xia Zhang 1 , Hong‐Liang Xu 1 , Zhong‐Min Su 1, 2
Affiliation
|
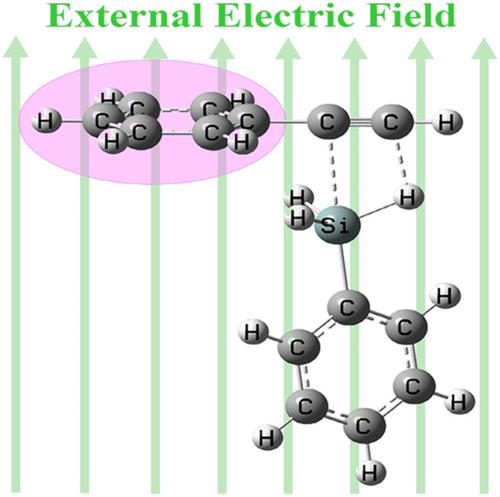
The hydrosilylation of terminal alkyne is the most straightforward and atom‐economy method to generate α‐vinylsilane. In the present paper, the acetylene and phenylacetylene hydrosilylation reactions were firstly studied by imposing external electric field (EEF) as a catalyst at the level of ωB97XD/TZVP. The results demonstrated that the oriented negative FY direction is the optimal direction, which mostly reduced the reaction barriers. Further computations indicated that the larger the EEF, the lower the barrier. When the EEF was increased to 180 (10−4) au, the barrier height of acetylene hydrosilylation (path Q) reduced almost 20 kcal/mol. To be surprised, as the phenyl substitute used, the hydrosilylation reaction (path FQ) was largely accelerated with the barrier height lowered to 13.2 kcal/mol, which decreased about 37 kcal/mol. Hopefully, this theoretical study would provide a guide for the experiment of the hydrosilylation of alkyne as much as possible.
中文翻译:

外部电场—苯基相互作用促进了取代炔烃向α-乙烯基硅烷的氢化硅烷化
末端炔烃的氢化硅烷化是生成α-乙烯基硅烷的最简单,最经济的方法。本文首先以ωB97XD/ TZVP为外加电场作为催化剂,研究了乙炔和苯乙炔的硅氢加成反应。结果表明,取向的负FY方向是最佳方向,主要减少了反应壁垒。进一步的计算表明,EEF越大,势垒越低。当EEF增加到180(10 -4)au,乙炔氢化硅烷化的势垒高度(路径Q)降低了近20 kcal / mol。令人惊讶的是,作为所用的苯基取代基,氢化硅烷化反应(路径FQ)大大加速,势垒高度降低至13.2 kcal / mol,降低了约37 kcal / mol。希望该理论研究能够为炔烃的氢化硅烷化实验提供指导。
更新日期:2020-02-18
中文翻译:

外部电场—苯基相互作用促进了取代炔烃向α-乙烯基硅烷的氢化硅烷化
末端炔烃的氢化硅烷化是生成α-乙烯基硅烷的最简单,最经济的方法。本文首先以ωB97XD/ TZVP为外加电场作为催化剂,研究了乙炔和苯乙炔的硅氢加成反应。结果表明,取向的负FY方向是最佳方向,主要减少了反应壁垒。进一步的计算表明,EEF越大,势垒越低。当EEF增加到180(10 -4)au,乙炔氢化硅烷化的势垒高度(路径Q)降低了近20 kcal / mol。令人惊讶的是,作为所用的苯基取代基,氢化硅烷化反应(路径FQ)大大加速,势垒高度降低至13.2 kcal / mol,降低了约37 kcal / mol。希望该理论研究能够为炔烃的氢化硅烷化实验提供指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号