当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure, Magnetism, and Electrochemistry of LiMg1-xZnxVO4 Spinels with 0 ≤ x ≤ 1.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.inorgchem.9b03058 Takeshi Uyama 1 , Kazuhiko Mukai 1 , Ikuya Yamada 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.inorgchem.9b03058 Takeshi Uyama 1 , Kazuhiko Mukai 1 , Ikuya Yamada 2
Affiliation
|
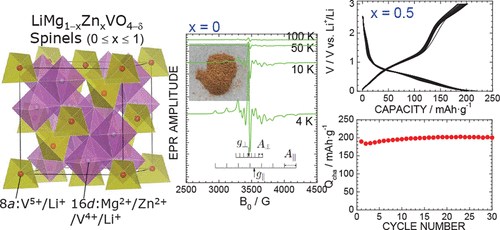
Negative electrode materials with lower operating voltages are urgently required to increase the energy density of lithium-ion batteries. In this study, LiMgVO4 with a Na2CrO4-type structure, LiZnVO4 with a phenacite structure, and their mixture were treated under a high pressure of 12 GPa and a high temperature of 1273 K, and their electrochemical reactivities were examined in a nonaqueous lithium cell. Synchrotron X-ray diffraction (XRD) measurements and Raman spectroscopy revealed that the LiMg1-xZnxVO4 samples with 0 ≤ x ≤ 1 are in a single phase of the inverse spinel structure that forms a solid solution compound over the whole x range. All of the samples were brown or light black due to the presence of a small amount of V4+ ions with S = 1/2 and oxygen deficiencies. Since the majority of the vanadium ions are located at the route of the Li+ ion conduction pathway, no rechargeable capacity (Qrecha) would be expected. Nevertheless, all LiMg1-xZnxVO4 samples exhibited a Qrecha value of more than 200 mAh g-1 with an operating voltage of ∼0.8 V. This operating voltage is ∼1.6 V lower than that of LiV2O4 with a normal spinel structure. Furthermore, the x = 0.5 sample demonstrated an extremely stable cycle performance over 1 month. Ex situ XRD measurements clarified that the reversible electrochemical reaction can be attributed to the movement of vanadium ions from the tetrahedral 8a to octahedral 16c sites during the initial discharge reaction. Details regarding the crystal structure, magnetism, and electrochemistry of LiMg1-xZnxVO4 are presented.
中文翻译:

0≤x≤1的LiMg1-xZnxVO4尖晶石的结构,磁性和电化学。
迫切需要具有较低工作电压的负极材料,以提高锂离子电池的能量密度。在这项研究中,具有Na2CrO4型结构的LiMgVO4,具有方铅矿结构的LiZnVO4及其混合物在12 GPa的高压和1273 K的高温下进行了处理,并在非水锂电池中检查了它们的电化学反应性。同步加速器X射线衍射(XRD)测量和拉曼光谱显示,0≤x≤1的LiMg1-xZnxVO4样品位于反尖晶石结构的单相中,在整个x范围内形成固溶体化合物。由于存在少量的S = 1/2的V4 +离子和缺氧,所有样品均为棕色或浅黑色。由于大多数钒离子位于Li +离子传导途径的路径上,因此不会产生可充电容量(Qrecha)。然而,所有LiMg1-xZnxVO4样品在约0.8 V的工作电压下均表现出超过200 mAh g-1的Qrecha值。该工作电压比具有正常尖晶石结构的LiV2O4的Qrecha值低约1.6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。没有可充电容量(Qrecha)。然而,所有LiMg1-xZnxVO4样品在约0.8 V的工作电压下均表现出超过200 mAh g-1的Qrecha值。该工作电压比具有正常尖晶石结构的LiV2O4的Qrecha值低约1.6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。没有可充电容量(Qrecha)。然而,所有LiMg1-xZnxVO4样品在约0.8 V的工作电压下均表现出超过200 mAh g-1的Qrecha值。该工作电压比具有正常尖晶石结构的LiV2O4的Qrecha值低约1.6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。比具有正常尖晶石结构的LiV2O4低6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。比具有正常尖晶石结构的LiV2O4低6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。
更新日期:2019-12-17
中文翻译:

0≤x≤1的LiMg1-xZnxVO4尖晶石的结构,磁性和电化学。
迫切需要具有较低工作电压的负极材料,以提高锂离子电池的能量密度。在这项研究中,具有Na2CrO4型结构的LiMgVO4,具有方铅矿结构的LiZnVO4及其混合物在12 GPa的高压和1273 K的高温下进行了处理,并在非水锂电池中检查了它们的电化学反应性。同步加速器X射线衍射(XRD)测量和拉曼光谱显示,0≤x≤1的LiMg1-xZnxVO4样品位于反尖晶石结构的单相中,在整个x范围内形成固溶体化合物。由于存在少量的S = 1/2的V4 +离子和缺氧,所有样品均为棕色或浅黑色。由于大多数钒离子位于Li +离子传导途径的路径上,因此不会产生可充电容量(Qrecha)。然而,所有LiMg1-xZnxVO4样品在约0.8 V的工作电压下均表现出超过200 mAh g-1的Qrecha值。该工作电压比具有正常尖晶石结构的LiV2O4的Qrecha值低约1.6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。没有可充电容量(Qrecha)。然而,所有LiMg1-xZnxVO4样品在约0.8 V的工作电压下均表现出超过200 mAh g-1的Qrecha值。该工作电压比具有正常尖晶石结构的LiV2O4的Qrecha值低约1.6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。没有可充电容量(Qrecha)。然而,所有LiMg1-xZnxVO4样品在约0.8 V的工作电压下均表现出超过200 mAh g-1的Qrecha值。该工作电压比具有正常尖晶石结构的LiV2O4的Qrecha值低约1.6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。比具有正常尖晶石结构的LiV2O4低6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。比具有正常尖晶石结构的LiV2O4低6V。此外,x = 0.5的样品在1个月内表现出极其稳定的循环性能。异位XRD测量表明,可逆的电化学反应可归因于初始放电反应过程中钒离子从四面体8a到八面体16c的运动。给出了有关LiMg1-xZnxVO4的晶体结构,磁性和电化学的详细信息。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号