当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engaging Sulfonamides: Intramolecular Cross-Electrophile Coupling Reaction of Sulfonamides with Alkyl Chlorides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.joc.9b02603 Erika L Lucas 1 , Kirsten A Hewitt 1 , Pan-Pan Chen 2 , Anthony J Castro 1 , Xin Hong 2 , Elizabeth R Jarvo 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.joc.9b02603 Erika L Lucas 1 , Kirsten A Hewitt 1 , Pan-Pan Chen 2 , Anthony J Castro 1 , Xin Hong 2 , Elizabeth R Jarvo 1
Affiliation
|
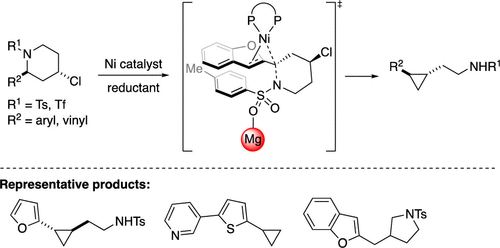
The application of amine derivatives as coupling partners is rare due to the inherent strength of the C-N bond. Herein, we report the first cross-electrophile coupling reaction of unstrained benzylic sulfonamides. Nickel-catalyzed intramolecular cross-electrophile coupling reactions of acyclic and cyclic benzylic sulfonamides with pendant alkyl chlorides generate cyclopropane products. Mechanistic experiments and DFT calculations are consistent with initiation of the reaction by magnesium iodide accelerated oxidative addition of the benzylic sulfonamide. This work establishes neutral and unstrained amine derivatives as XEC partners, furnishes structural rearrangement of benzylic sulfonamides, and provides valuable information regarding catalyst design for the development of new cross-electrophile coupling reactions of carbon-heteroatom bonds.
中文翻译:

参与磺酰胺:磺酰胺与烷基氯化物的分子内跨亲电偶联反应。
由于CN键的固有强度,很少使用胺衍生物作为偶联伴侣。在这里,我们报告了未应变的苄基磺酰胺的第一个交叉亲电子偶联反应。无环和环状苄基磺酰胺与烷基氯化侧基的镍催化的分子内亲电偶联反应生成环丙烷产物。机理实验和DFT计算与通过碘化镁加速苄基磺酰胺的氧化加成反应的反应是一致的。这项工作建立了作为XEC伙伴的中性和非应变胺衍生物,提供了苄基磺酰胺的结构重排,并提供了有关催化剂设计的有价值的信息,以开发新的碳-杂原子键交叉亲电子偶联反应。
更新日期:2019-12-17
中文翻译:

参与磺酰胺:磺酰胺与烷基氯化物的分子内跨亲电偶联反应。
由于CN键的固有强度,很少使用胺衍生物作为偶联伴侣。在这里,我们报告了未应变的苄基磺酰胺的第一个交叉亲电子偶联反应。无环和环状苄基磺酰胺与烷基氯化侧基的镍催化的分子内亲电偶联反应生成环丙烷产物。机理实验和DFT计算与通过碘化镁加速苄基磺酰胺的氧化加成反应的反应是一致的。这项工作建立了作为XEC伙伴的中性和非应变胺衍生物,提供了苄基磺酰胺的结构重排,并提供了有关催化剂设计的有价值的信息,以开发新的碳-杂原子键交叉亲电子偶联反应。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号