当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
S-(4-Methoxyphenyl)-4-methoxybenzenesulfonothioate as a Promising Lead Compound for the Development of a Renal Carcinoma Agent.
ChemMedChem ( IF 3.6 ) Pub Date : 2019-12-13 , DOI: 10.1002/cmdc.201900566 Camilla I Nantes 1 , Ingrid D Pereira 2 , Ruoli Bai 3 , Ernest Hamel 3 , James C Burnett 4 , Rodrigo J de Oliveira 5 , Maria de F C Matos 1 , Adilson Beatriz 2 , Murilo K A Yonekawa 6 , Renata T Perdomo 1 , Dênis P de Lima 2 , Danielle Bogo 1 , Edson Dos A Dos Santos 6
ChemMedChem ( IF 3.6 ) Pub Date : 2019-12-13 , DOI: 10.1002/cmdc.201900566 Camilla I Nantes 1 , Ingrid D Pereira 2 , Ruoli Bai 3 , Ernest Hamel 3 , James C Burnett 4 , Rodrigo J de Oliveira 5 , Maria de F C Matos 1 , Adilson Beatriz 2 , Murilo K A Yonekawa 6 , Renata T Perdomo 1 , Dênis P de Lima 2 , Danielle Bogo 1 , Edson Dos A Dos Santos 6
Affiliation
|
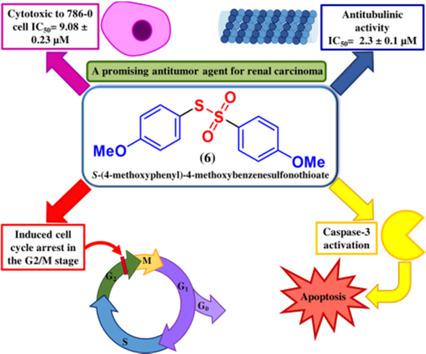
Organosulfur compounds show cytotoxic potential towards many tumor cell lines. Disulfides and thiosulfonates act through apoptotic processes, inducing proteins associated with apoptosis, endoplasmic reticulum stress, and the unfolded protein response. Three p-substituted symmetric diaryl disulfides and three diaryl thiosulfonates were synthesized and analyzed for inhibition of tubulin polymerization and for human cancer cell cytotoxic activity against seven tumor cell lines and a non-tumor cell line. S-(4-methoxyphenyl)-4-methoxybenzenesulfonothioate (6) exhibited inhibition of tubulin polymerization and showed the best antiproliferative potential, especially against the 786-0 cell line, being six times more selective as compared with the non-tumor cell line. In addition, compound 6 was able to activate caspase-3 after 24 and 48 h treatments of the 786-0 cell line and induced cell-cycle arrest in the G2/M stage at the highest concentration evaluated at 24 and 48 h. Compound 6 was able to cause complete inhibition of proliferation, inducing the death of 786-0 cells, by increasing the number of cells at G2/M and greater activation of caspase-3.
中文翻译:

S-(4-甲氧基苯基)-4-甲氧基苯硫代硫酸盐作为开发肾癌药物的有前途的先导化合物。
有机硫化合物对许多肿瘤细胞系显示出细胞毒性潜力。二硫化物和硫代磺酸盐通过凋亡过程起作用,诱导与凋亡,内质网应激和未折叠的蛋白质反应相关的蛋白质。合成了三种对位取代的对称二芳基二硫化物和三种二芳基硫代磺酸盐,并分析了其对微管蛋白聚合的抑制作用以及对七种肿瘤细胞系和非肿瘤细胞系的人癌细胞毒性。S-(4-甲氧基苯基)-4-甲氧基苯硫代硫酸盐(6)表现出对微管蛋白聚合的抑制作用,并显示出最佳的抗增殖潜力,尤其是对786-0细胞系的选择性,是非肿瘤细胞系的六倍。此外,在对786-0细胞系进行24和48小时处理后,化合物6能够激活caspase-3,并在24/48 h评估的最高浓度下在G2 / M期诱导细胞周期停滞。通过增加G2 / M处的细胞数量和caspase-3的更大活化,化合物6能够完全抑制增殖,诱导786-0细胞死亡。
更新日期:2020-01-17
中文翻译:

S-(4-甲氧基苯基)-4-甲氧基苯硫代硫酸盐作为开发肾癌药物的有前途的先导化合物。
有机硫化合物对许多肿瘤细胞系显示出细胞毒性潜力。二硫化物和硫代磺酸盐通过凋亡过程起作用,诱导与凋亡,内质网应激和未折叠的蛋白质反应相关的蛋白质。合成了三种对位取代的对称二芳基二硫化物和三种二芳基硫代磺酸盐,并分析了其对微管蛋白聚合的抑制作用以及对七种肿瘤细胞系和非肿瘤细胞系的人癌细胞毒性。S-(4-甲氧基苯基)-4-甲氧基苯硫代硫酸盐(6)表现出对微管蛋白聚合的抑制作用,并显示出最佳的抗增殖潜力,尤其是对786-0细胞系的选择性,是非肿瘤细胞系的六倍。此外,在对786-0细胞系进行24和48小时处理后,化合物6能够激活caspase-3,并在24/48 h评估的最高浓度下在G2 / M期诱导细胞周期停滞。通过增加G2 / M处的细胞数量和caspase-3的更大活化,化合物6能够完全抑制增殖,诱导786-0细胞死亡。































 京公网安备 11010802027423号
京公网安备 11010802027423号