Chemosphere ( IF 8.1 ) Pub Date : 2019-12-16 , DOI: 10.1016/j.chemosphere.2019.125628 Jinghua Zhang , Huangbo Chen , Huan He , Xinying Cheng , Tao Ma , Jiapeng Hu , Shaogui Yang , Shiyin Li , Limin Zhang
|
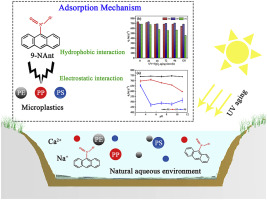
Microplastics and Nitropolycyclic aromatic hydrocarbons (NPAHs) are two types of emerging pollutants that are strong potential threats to aquatic ecosystems and organisms. The adsorption of NPAHs on microplastics may explain the fate and effects of NPAHs in natural environments. In this study, the adsorption behavior of 9-Nitroanthrene (9-NAnt) on polyethylene (PE), polypropylene (PP) and polystyrene (PS) was investigated. Kinetic experiments revealed that 9-NAnt was inclined to be adsorbed onto microplastics, especially PE, which had a large adsorption amount of 734 μg g−1. A linear isothermal model better described the isothermal adsorption process for 9-NAnt, which indicated that a hydrophobic distribution may be the main adsorption mechanism in an aqueous solution. Water environment factors, such as the pH and ionic strength, had negligible effects on the adsorption for PE. In contrast, alkaline and high ionic strength conditions resulted in the inhibition of adsorption of PP and PS. In addition, the particle size of microplastics was negatively correlated with the log Kd of 9-NAnt, and the performance of transient aging treatments on microplastics reduced their affinity for 9-NAnt, due to the addition of oxygen-containing functional groups. Above all, hydrophobic and electrostatic processes were the main adsorption mechanisms between microplastics and 9-NAnt.
中文翻译:

9-硝基蒽在典型微塑料中的吸附行为及其机理
微塑料和硝基多环芳烃(NPAHs)是两种新兴污染物,它们对水生生态系统和生物体具有强大的潜在威胁。NPAHs在微塑料上的吸附可能解释了NPAHs在自然环境中的命运和影响。在这项研究中,研究了9-硝基蒽(9-NAnt)在聚乙烯(PE),聚丙烯(PP)和聚苯乙烯(PS)上的吸附行为。动力学实验表明,9-NAnt倾向于吸附在微塑料上,尤其是PE,其吸附量大,为734μgg -1。线性等温模型较好地描述了9-NAnt的等温吸附过程,这表明疏水性分布可能是水溶液中的主要吸附机理。pH值和离子强度等水环境因素对PE的吸附影响可忽略不计。相反,碱性和高离子强度条件导致抑制PP和PS的吸附。此外,微塑料的粒径与9-NAnt的log K d负相关,由于添加了含氧官能团,瞬态老化处理对微塑料的性能降低了它们对9-NAnt的亲和力。最重要的是,疏水和静电过程是微塑料与9-NAnt之间的主要吸附机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号