当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Dual Role of Lithium Difluoro(oxalato)borate Additive in Improving the Electrochemical Performance of NMC811||Graphite Cells
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-12-13 00:00:00 , DOI: 10.1021/acsaem.9b01894 Qingyu Dong 1, 2 , Feng Guo 1, 2 , Zhenjie Cheng 1, 2 , Yayun Mao 2 , Rong Huang 3 , Fangsen Li 3 , Houcai Dong 2 , Qingyong Zhang 4 , Wei Li 4 , Hui Chen 4 , Zhaojun Luo 4 , Yanbin Shen 2 , Xiaodong Wu 2 , Liwei Chen 2, 3, 5
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-12-13 00:00:00 , DOI: 10.1021/acsaem.9b01894 Qingyu Dong 1, 2 , Feng Guo 1, 2 , Zhenjie Cheng 1, 2 , Yayun Mao 2 , Rong Huang 3 , Fangsen Li 3 , Houcai Dong 2 , Qingyong Zhang 4 , Wei Li 4 , Hui Chen 4 , Zhaojun Luo 4 , Yanbin Shen 2 , Xiaodong Wu 2 , Liwei Chen 2, 3, 5
Affiliation
|
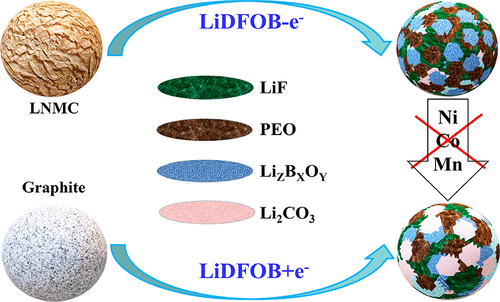
Ni-rich layered oxides (LiNixMnyCozO2, x ≥ 0.6, x + y + z = 1) are promising positive electrode materials for high energy density lithium-ion batteries thanks to their high specific capacity. However, large-scale application of Ni-rich layered oxides is hindered by its poor structural and interfacial stability, especially during cycling at a high cutoff potential (i.e., ≥ 4.3 V, versus Li+/Li). Herein, we demonstrate that lithium difluoro(oxalato)borate (LiDFOB) as a film-forming additive plays a dual role on the electrode|electrolyte interphase formation in a LiNi0.83Mn0.05Co0.12O2||graphite cell, meaning that it can not only be reduced on the graphite negative electrode but also oxidized on the nickel-rich oxide LiNi0.83Mn0.05Co0.12O2 positive electrode cycled at a high cutoff potential (4.4 V, versus Li+/Li) prior to typical carbonate-based electrolyte constituents. As a result, the addition of 1.5 wt % LiDFOB greatly reduces the polarization and improves the cycling stability of the LiNi0.83Mn0.05Co0.12O2||graphite cell, which shows a high discharge capacity of 198 mA h g–1, and more than 83.1% of the initial capacity was retained after 200 cycles at C/3 (the capacity retention obtained at the same cycling condition is only 59.9% for the cell without LiDFOB additive). Furthermore, the employ of LiDFOB additive also significantly suppresses the self-discharge of the LiNi0.83Mn0.05Co0.12O2||Li cell during high-temperature and long-term room-temperature storage at 4.4 V. These electrochemical performance enhancements could be attributed to the participation of LiDFOB in forming a stable and Li+ transfer favorable protective layer that is rich in inorganic boron, fluorine, and carbonate compounds on both the surface of the LiNi0.83Mn0.05Co0.12O2 positive electrode and the graphite negative electrode, thus suppressing the electrolyte decomposition on the positive electrode and negative electrode surfaces and decreasing the dissolution of transition-metal ions from the positive electrode bulk.
中文翻译:

洞察二氟(草酸)硼酸锂添加剂在改善NMC811 ||石墨电池电化学性能中的双重作用
富Ni层状氧化物(的LiNi X锰ý钴Ž ø 2,X ≥0.6,X + ÿ + Ž = 1)是有希望的由于高能量密度的锂离子电池的正极材料它们的高比容量。但是,富镍层状氧化物的大规模应用因其不良的结构和界面稳定性而受到阻碍,尤其是在高截止电位(即,相对于Li + / Li≥4.3 V)循环时。在此,我们证明了作为成膜添加剂的二氟(草酸硼酸)硼酸锂(LiDFOB)在LiNi 0.83 Mn中的电解质界面形成中起双重作用。0.05 Co 0.12 O 2 ||石墨电池,这意味着它不仅可以在石墨负极上还原,而且可以在高截止电位(4.4循环)的富镍氧化物LiNi 0.83 Mn 0.05 Co 0.12 O 2正极上被氧化V,相对于Li + / Li),先于典型的基于碳酸盐的电解质成分。结果,添加1.5 wt%的LiDFOB大大降低了极化并改善了LiNi 0.83 Mn 0.05 Co 0.12 O 2 ||石墨电池的循环稳定性,显示了198 mA hg –1的高放电容量。,并且在200次循环后以C / 3保留了超过83.1%的初始容量(对于没有LiDFOB添加剂的电池,在相同循环条件下获得的容量保留率仅为59.9%)。此外,使用LiDFOB添加剂还可以显着抑制LiNi 0.83 Mn 0.05 Co 0.12 O 2 || Li电池在4.4 V的高温和长期室温存储过程中的自放电。这些电化学性能的提高可能是归因于LiDFOB的参与形成稳定和Li +转印良好的保护层,其含有丰富的无机硼,氟和所述的LiNi的两个表面上的碳酸酯化合物0.83锰0.05Co 0.12 O 2正极和石墨负极,因此抑制了电解质在正极和负极表面上的分解,并减少了过渡金属离子从正极块中的溶解。
更新日期:2019-12-13
中文翻译:

洞察二氟(草酸)硼酸锂添加剂在改善NMC811 ||石墨电池电化学性能中的双重作用
富Ni层状氧化物(的LiNi X锰ý钴Ž ø 2,X ≥0.6,X + ÿ + Ž = 1)是有希望的由于高能量密度的锂离子电池的正极材料它们的高比容量。但是,富镍层状氧化物的大规模应用因其不良的结构和界面稳定性而受到阻碍,尤其是在高截止电位(即,相对于Li + / Li≥4.3 V)循环时。在此,我们证明了作为成膜添加剂的二氟(草酸硼酸)硼酸锂(LiDFOB)在LiNi 0.83 Mn中的电解质界面形成中起双重作用。0.05 Co 0.12 O 2 ||石墨电池,这意味着它不仅可以在石墨负极上还原,而且可以在高截止电位(4.4循环)的富镍氧化物LiNi 0.83 Mn 0.05 Co 0.12 O 2正极上被氧化V,相对于Li + / Li),先于典型的基于碳酸盐的电解质成分。结果,添加1.5 wt%的LiDFOB大大降低了极化并改善了LiNi 0.83 Mn 0.05 Co 0.12 O 2 ||石墨电池的循环稳定性,显示了198 mA hg –1的高放电容量。,并且在200次循环后以C / 3保留了超过83.1%的初始容量(对于没有LiDFOB添加剂的电池,在相同循环条件下获得的容量保留率仅为59.9%)。此外,使用LiDFOB添加剂还可以显着抑制LiNi 0.83 Mn 0.05 Co 0.12 O 2 || Li电池在4.4 V的高温和长期室温存储过程中的自放电。这些电化学性能的提高可能是归因于LiDFOB的参与形成稳定和Li +转印良好的保护层,其含有丰富的无机硼,氟和所述的LiNi的两个表面上的碳酸酯化合物0.83锰0.05Co 0.12 O 2正极和石墨负极,因此抑制了电解质在正极和负极表面上的分解,并减少了过渡金属离子从正极块中的溶解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号