当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Structure and Stability of Ammonium Azide Under High Pressure
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.jpcc.9b09635 Guozhao Zhang 1, 2 , Haiwa Zhang 2 , Sandra Ninet 2 , Hongyang Zhu 3 , Cailong Liu 1, 4 , Jean-Paul Itié 5 , Chunxiao Gao 1 , Frédéric Datchi 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.jpcc.9b09635 Guozhao Zhang 1, 2 , Haiwa Zhang 2 , Sandra Ninet 2 , Hongyang Zhu 3 , Cailong Liu 1, 4 , Jean-Paul Itié 5 , Chunxiao Gao 1 , Frédéric Datchi 2
Affiliation
|
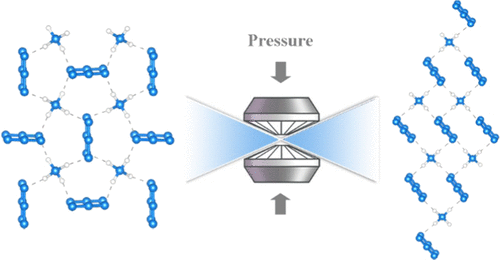
Due to its potential applications as a high-energy density material, the high-pressure polymorphs of ammonium azide (AA) have received much attention recently. However, the crystal structure of phase II (AA-II), stable above 3.0 GPa, has remained elusive until now. By combining X-ray diffraction and Raman experiments with first-principles calculations, we determine that AA-II is a hydrogen (H)-bonded structure of ammonium and azide ions with monoclinic symmetry, space group P2/c. The latter is comprised of alternating molecular layers of ammonium and azide ions and mostly differs from AA-I by its denser packing of molecular planes while preserving the hydrogen bond network. First-principles calculations show that phase II has the lowest enthalpy among all other considered structures from 4.9 to 102.6 GPa, pushing the phase transitions to the previously predicted hydronitrogen solids to higher pressures. Raman data to 85.0 GPa at room temperature confirm the absence of phase transition and agree very well with the pressure evolution of the Raman modes of AA-II predicted by our calculations.
中文翻译:

高压下叠氮化铵的晶体结构和稳定性
由于其作为高能量密度材料的潜在应用,叠氮化铵(AA)的高压多晶型物最近受到了广泛关注。然而,直到现在仍稳定在3.0 GPa以上的II相(AA-II)晶体结构。通过将X射线衍射和拉曼实验与第一性原理相结合,我们确定AA-II是单斜对称,空间群P 2 / c的氢(H)键结构的铵离子和叠氮化物离子。。后者由铵和叠氮化物离子的交替分子层组成,并且与AA-I的主要不同之处在于它在保持氢键网络的同时更紧密地堆积了分子平面。第一性原理计算表明,在所有其他考虑的结构中,II相具有最低的焓,范围从4.9 GPa到102.6 GPa,这将使先前预测的氢氮固体的相变推至更高的压力。在室温下达到85.0 GPa的拉曼数据证实了不存在相变,并且与我们的计算预测的AA-II拉曼模式的压力变化非常吻合。
更新日期:2019-12-27
中文翻译:

高压下叠氮化铵的晶体结构和稳定性
由于其作为高能量密度材料的潜在应用,叠氮化铵(AA)的高压多晶型物最近受到了广泛关注。然而,直到现在仍稳定在3.0 GPa以上的II相(AA-II)晶体结构。通过将X射线衍射和拉曼实验与第一性原理相结合,我们确定AA-II是单斜对称,空间群P 2 / c的氢(H)键结构的铵离子和叠氮化物离子。。后者由铵和叠氮化物离子的交替分子层组成,并且与AA-I的主要不同之处在于它在保持氢键网络的同时更紧密地堆积了分子平面。第一性原理计算表明,在所有其他考虑的结构中,II相具有最低的焓,范围从4.9 GPa到102.6 GPa,这将使先前预测的氢氮固体的相变推至更高的压力。在室温下达到85.0 GPa的拉曼数据证实了不存在相变,并且与我们的计算预测的AA-II拉曼模式的压力变化非常吻合。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号