当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capture of Iodide by Bismuth Vanadate and Bismuth Oxide: An Insight into the Process and its Aftermath
ChemSusChem ( IF 7.5 ) Pub Date : 2018-04-17 , DOI: 10.1002/cssc.201800327 Liping Zhang 1 , Alexandre A. S. Gonçalves 1 , Baojiang Jiang 1, 2 , Mietek Jaroniec 1
ChemSusChem ( IF 7.5 ) Pub Date : 2018-04-17 , DOI: 10.1002/cssc.201800327 Liping Zhang 1 , Alexandre A. S. Gonçalves 1 , Baojiang Jiang 1, 2 , Mietek Jaroniec 1
Affiliation
|
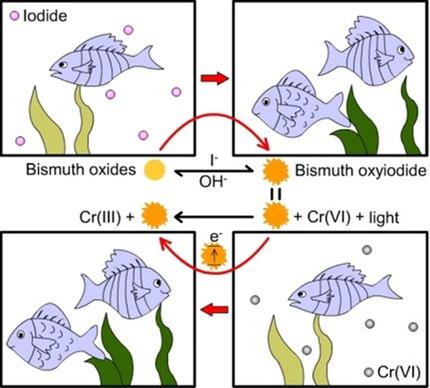
As a result of the scarcity of iodine, as well as its threat to the environment if it is present in excess, iodine as a waste needs to be captured. Compared with ion‐exchange resins and Ag‐containing materials, which are popular iodide adsorbents, Bi‐containing compounds show some important advantages, such as high iodide‐capture capacity and fast kinetics. In this study, two Bi‐containing compounds, BiVO4 and Bi2O3, were investigated comprehensively for iodide immobilization. The influence of the pH, iodide/adsorbent ratio, temperature, crystallite size, and competing ions was explored, with a view to optimization of the capture process. Further study of the iodide‐adsorbed bismuth compounds confirms that the capture of iodide by BiVO4 and Bi2O3 is a chemisorption process with the formation of bismuth oxyiodide (BixOyIz). Furthermore, iodide ions are able to penetrate into the bulk of BiVO4 and Bi2O3, which is believed to be responsible for their high capture capacity. The application of BixOyIz as a photocatalyst has also been examined in CrVI reduction. This result makes the capture of iodide by BiVO4 and Bi2O3 even more environmentally friendly as the photocatalytic application of the iodide‐containing adsorbents not only avoids the production of secondary waste but may help to solve other environmental issues.
中文翻译:

钒酸铋和氧化铋对碘化物的捕获:过程及其后果的见解
由于碘的缺乏以及碘的过量存在对环境的威胁,需要捕获碘作为废物。与流行的碘化物吸附剂离子交换树脂和含银材料相比,含铋化合物显示出一些重要的优势,例如高碘化物捕获能力和快速动力学。在这项研究中,对两种含Bi的化合物BiVO 4和Bi 2 O 3进行了全面的碘化物固定化研究。探讨了pH,碘化物/吸附剂比率,温度,微晶尺寸和竞争离子的影响,以优化捕获过程。对碘化物吸附的铋化合物的进一步研究证实,BiVO 4可以捕获碘化物Bi 2 O 3是化学吸附过程,形成碘氧化铋(Bi x O y I z)。此外,碘离子能够渗透到BiVO 4和Bi 2 O 3的主体中,这被认为是其高捕获能力的原因。还已经研究了在Cr VI还原中Bi x O y I z作为光催化剂的应用。该结果使得BiVO 4和Bi 2 O 3捕获碘化物。 由于对含碘化物的吸附剂进行光催化应用,因此更加环保,不仅避免了二次废物的产生,而且可能有助于解决其他环境问题。
更新日期:2018-04-17
中文翻译:

钒酸铋和氧化铋对碘化物的捕获:过程及其后果的见解
由于碘的缺乏以及碘的过量存在对环境的威胁,需要捕获碘作为废物。与流行的碘化物吸附剂离子交换树脂和含银材料相比,含铋化合物显示出一些重要的优势,例如高碘化物捕获能力和快速动力学。在这项研究中,对两种含Bi的化合物BiVO 4和Bi 2 O 3进行了全面的碘化物固定化研究。探讨了pH,碘化物/吸附剂比率,温度,微晶尺寸和竞争离子的影响,以优化捕获过程。对碘化物吸附的铋化合物的进一步研究证实,BiVO 4可以捕获碘化物Bi 2 O 3是化学吸附过程,形成碘氧化铋(Bi x O y I z)。此外,碘离子能够渗透到BiVO 4和Bi 2 O 3的主体中,这被认为是其高捕获能力的原因。还已经研究了在Cr VI还原中Bi x O y I z作为光催化剂的应用。该结果使得BiVO 4和Bi 2 O 3捕获碘化物。 由于对含碘化物的吸附剂进行光催化应用,因此更加环保,不仅避免了二次废物的产生,而且可能有助于解决其他环境问题。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号