当前位置:
X-MOL 学术
›
J. Biomed. Mater. Res. Part B Appl. Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved oral bioavailability of repaglinide, a typical BCS Class II drug, with a chitosan-coated nanoemulsion.
Journal of Biomedical Materials Research Part B: Applied Biomaterials ( IF 3.2 ) Pub Date : 2019-06-12 , DOI: 10.1002/jbm.b.34426 Zahra Karami 1, 2 , Mohammad Reza Saghatchi Zanjani 1, 2, 3 , Nadia Nasihatsheno 2, 4 , Mehrdad Hamidi 1, 2
Journal of Biomedical Materials Research Part B: Applied Biomaterials ( IF 3.2 ) Pub Date : 2019-06-12 , DOI: 10.1002/jbm.b.34426 Zahra Karami 1, 2 , Mohammad Reza Saghatchi Zanjani 1, 2, 3 , Nadia Nasihatsheno 2, 4 , Mehrdad Hamidi 1, 2
Affiliation
|
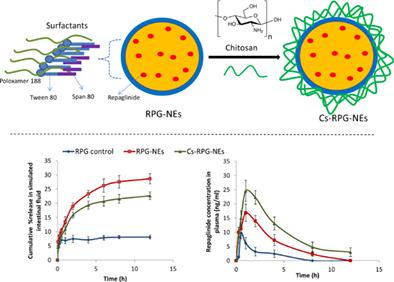
The aim of the present study was to develop modified nanoemulsions to improve the oral bioavailability and pharmacokinetics of a poor water‐soluble drug, repaglinide (RPG). The repaglinide‐loaded nanoemulsions (RPG‐NEs) were prepared from olive oil as internal phase, span 80, tween 80, and poloxamer 188 as emulsifiers, using homogenization technique. The mean droplet size, zeta potential, and entrapment efficiency of RPG‐NEs were 86.5 ± 3.4 nm, −33.8 ± 2.1 mV, and 96.3 ± 2.3%, respectively. The chitosan‐coated RPG‐NEs (Cs‐RPG‐NEs) showed an average droplet size of 149.3 ± 3.9 nm and a positive zeta‐potential of +31.5 ± 2.8 mV. Drug release profile of RPG‐NEs was significantly higher than free drug in the simulated gastrointestinal fluids (p < .005). The in vivo study revealed 3.51‐ and 1.78‐fold increase in the AUC0‐12h and Cmax of the drug, respectively, in RPG‐NEs‐receiving animals in comparison to the free drug. The pharmacokinetic analysis confirmed that Cs‐RPG‐NEs were more efficient than uncoated ones for the oral delivery of RPG. Cs‐RPG‐NEs showed a longer t1/2 and higher AUC0‐∞ compared to control group. The relative bioavailability of Cs‐RPG‐NEs was higher than that of uncoated RPG‐NEs and free drug. Collectively, these findings suggest that chitosan‐coated nanoemulsions are promising carrier for improving the oral bioavailability of RPG.
中文翻译:

使用壳聚糖涂层纳米乳剂提高瑞格列奈(一种典型的 BCS II 类药物)的口服生物利用度。
本研究的目的是开发改性纳米乳剂,以提高水溶性差的药物瑞格列奈 (RPG) 的口服生物利用度和药代动力学。以橄榄油为内相、跨度 80、吐温 80 和泊洛沙姆 188 为乳化剂,使用均质化技术制备了载有瑞格列奈的纳米乳剂 (RPG-NE)。RPG-NE 的平均液滴尺寸、zeta 电位和截留效率分别为 86.5 ± 3.4 nm、-33.8 ± 2.1 mV 和 96.3 ± 2.3%。壳聚糖包覆的RPG-NEs(Cs-RPG-NEs)的平均液滴尺寸为149.3±3.9nm,正zeta电位为+31.5±2.8mV。在模拟胃肠液中,RPG-NEs 的药物释放曲线显着高于游离药物(p < .005)。体内研究显示,与游离药物相比,接受 RPG- NEs 的动物中药物的 AUC 0-12h和C max分别增加了3.51倍和 1.78 倍。药代动力学分析证实,Cs-RPG-NEs 比未包衣的 RPG 口服给药更有效。与对照组相比,Cs-RPG-NEs 显示出更长的t 1/2和更高的 AUC 0-∞。Cs-RPG-NEs 的相对生物利用度高于未包衣的 RPG-NEs 和游离药物。总的来说,这些发现表明壳聚糖包覆的纳米乳剂是提高 RPG 口服生物利用度的有希望的载体。
更新日期:2019-06-12
中文翻译:

使用壳聚糖涂层纳米乳剂提高瑞格列奈(一种典型的 BCS II 类药物)的口服生物利用度。
本研究的目的是开发改性纳米乳剂,以提高水溶性差的药物瑞格列奈 (RPG) 的口服生物利用度和药代动力学。以橄榄油为内相、跨度 80、吐温 80 和泊洛沙姆 188 为乳化剂,使用均质化技术制备了载有瑞格列奈的纳米乳剂 (RPG-NE)。RPG-NE 的平均液滴尺寸、zeta 电位和截留效率分别为 86.5 ± 3.4 nm、-33.8 ± 2.1 mV 和 96.3 ± 2.3%。壳聚糖包覆的RPG-NEs(Cs-RPG-NEs)的平均液滴尺寸为149.3±3.9nm,正zeta电位为+31.5±2.8mV。在模拟胃肠液中,RPG-NEs 的药物释放曲线显着高于游离药物(p < .005)。体内研究显示,与游离药物相比,接受 RPG- NEs 的动物中药物的 AUC 0-12h和C max分别增加了3.51倍和 1.78 倍。药代动力学分析证实,Cs-RPG-NEs 比未包衣的 RPG 口服给药更有效。与对照组相比,Cs-RPG-NEs 显示出更长的t 1/2和更高的 AUC 0-∞。Cs-RPG-NEs 的相对生物利用度高于未包衣的 RPG-NEs 和游离药物。总的来说,这些发现表明壳聚糖包覆的纳米乳剂是提高 RPG 口服生物利用度的有希望的载体。































 京公网安备 11010802027423号
京公网安备 11010802027423号