当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methane Activation and Utilization: Current Status and Future Challenges
Energy Technology ( IF 3.6 ) Pub Date : 2019-11-20 , DOI: 10.1002/ente.201900826 Lanlan Sun 1 , Yu Wang 1 , Naijia Guan 1, 2 , Landong Li 1, 2
Energy Technology ( IF 3.6 ) Pub Date : 2019-11-20 , DOI: 10.1002/ente.201900826 Lanlan Sun 1 , Yu Wang 1 , Naijia Guan 1, 2 , Landong Li 1, 2
Affiliation
|
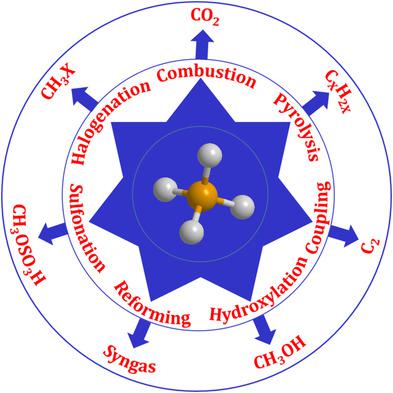
Methane, an abundant and clean fossil energy source, can provide the highest specific energy compared with other hydrocarbons. Exploiting an efficient way to convert methane to fuel or chemicals is, therefore, of significant importance economically as well as in terms of sustainability. Generally, methane is first converted to syngas in industry, which is further transformed to liquid hydrocarbons via a Fischer–Tropsch process. Methane is also directly pyrolyzed to olefins or aromatic hydrocarbons or coupled to C2 products at very high temperatures. Among all the direct routes, the selective oxidation of methane to methanol is noteworthy as it is thermodynamically favorable under relatively mild reaction conditions. However, restricted by the high C—H bond energy, the activation and utilization of methane still encounters great challenges despite the developments in recent years. Based on this background, this Review highlights the recent advances and forthcoming challenges in the process of methane activation and utilization. Catalysis plays a key role in the process of methane activation and it is focused on here. This Review is expected to be useful for scientists and engineers working in this field and is timely to boost further research.
中文翻译:

甲烷活化与利用:现状与未来挑战
甲烷是一种丰富而清洁的化石能源,与其他碳氢化合物相比,甲烷可提供最高的比能。因此,开发一种有效的方法将甲烷转化为燃料或化学物质在经济上和可持续性方面都具有重要意义。通常,甲烷首先在工业上转化为合成气,然后通过费-托工艺进一步转化为液态烃。甲烷也直接热解为烯烃或芳烃或与C 2偶联产品在很高的温度下。在所有直接途径中,值得注意的是甲烷选择性氧化为甲醇,因为在相对温和的反应条件下,甲烷在热力学上是有利的。然而,尽管近年来有了发展,但受到高CH键能的限制,甲烷的活化和利用仍然面临着巨大的挑战。基于此背景,本综述重点介绍了甲烷活化和利用过程中的最新进展和即将面临的挑战。催化在甲烷活化过程中起关键作用,在此重点介绍。预期该评论对从事该领域的科学家和工程师将是有用的,并且适时地促进了进一步的研究。
更新日期:2019-11-20
中文翻译:

甲烷活化与利用:现状与未来挑战
甲烷是一种丰富而清洁的化石能源,与其他碳氢化合物相比,甲烷可提供最高的比能。因此,开发一种有效的方法将甲烷转化为燃料或化学物质在经济上和可持续性方面都具有重要意义。通常,甲烷首先在工业上转化为合成气,然后通过费-托工艺进一步转化为液态烃。甲烷也直接热解为烯烃或芳烃或与C 2偶联产品在很高的温度下。在所有直接途径中,值得注意的是甲烷选择性氧化为甲醇,因为在相对温和的反应条件下,甲烷在热力学上是有利的。然而,尽管近年来有了发展,但受到高CH键能的限制,甲烷的活化和利用仍然面临着巨大的挑战。基于此背景,本综述重点介绍了甲烷活化和利用过程中的最新进展和即将面临的挑战。催化在甲烷活化过程中起关键作用,在此重点介绍。预期该评论对从事该领域的科学家和工程师将是有用的,并且适时地促进了进一步的研究。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号