Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease
Nature ( IF 50.5 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41586-019-1828-5
Najoua Lalaoui 1, 2 , Steven E Boyden 3 , Hirotsugu Oda 3 , Geryl M Wood 3 , Deborah L Stone 3 , Diep Chau 1 , Lin Liu 1, 2 , Monique Stoffels 3 , Tobias Kratina 1 , Kate E Lawlor 4, 5 , Kristien J M Zaal 6 , Patrycja M Hoffmann 3 , Nima Etemadi 1, 2 , Kristy Shield-Artin 1, 2 , Christine Biben 1, 2 , Wanxia Li Tsai 7 , Mary D Blake 7 , Hye Sun Kuehn 8 , Dan Yang 9 , Holly Anderton 1, 2 , Natasha Silke 1 , Laurens Wachsmuth 10, 11 , Lixin Zheng 12 , Natalia Sampaio Moura 3 , David B Beck 3 , Gustavo Gutierrez-Cruz 13 , Amanda K Ombrello 3 , Gineth P Pinto-Patarroyo 3 , Andrew J Kueh 1, 2 , Marco J Herold 1, 2 , Cathrine Hall 1 , Hongying Wang 3 , Jae Jin Chae 3 , Natalia I Dmitrieva 9 , Mark McKenzie 1, 2 , Amanda Light 1 , Beverly K Barham 3 , Anne Jones 3 , Tina M Romeo 3 , Qing Zhou 3 , Ivona Aksentijevich 3 , James C Mullikin 14 , Andrew J Gross 15 , Anthony K Shum 16 , Edwin D Hawkins 1, 2 , Seth L Masters 1, 2 , Michael J Lenardo 12 , Manfred Boehm 9 , Sergio D Rosenzweig 8 , Manolis Pasparakis 10, 11 , Anne K Voss 1, 2 , Massimo Gadina 7 , Daniel L Kastner 3 , John Silke 1, 2
Nature ( IF 50.5 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41586-019-1828-5
Najoua Lalaoui 1, 2 , Steven E Boyden 3 , Hirotsugu Oda 3 , Geryl M Wood 3 , Deborah L Stone 3 , Diep Chau 1 , Lin Liu 1, 2 , Monique Stoffels 3 , Tobias Kratina 1 , Kate E Lawlor 4, 5 , Kristien J M Zaal 6 , Patrycja M Hoffmann 3 , Nima Etemadi 1, 2 , Kristy Shield-Artin 1, 2 , Christine Biben 1, 2 , Wanxia Li Tsai 7 , Mary D Blake 7 , Hye Sun Kuehn 8 , Dan Yang 9 , Holly Anderton 1, 2 , Natasha Silke 1 , Laurens Wachsmuth 10, 11 , Lixin Zheng 12 , Natalia Sampaio Moura 3 , David B Beck 3 , Gustavo Gutierrez-Cruz 13 , Amanda K Ombrello 3 , Gineth P Pinto-Patarroyo 3 , Andrew J Kueh 1, 2 , Marco J Herold 1, 2 , Cathrine Hall 1 , Hongying Wang 3 , Jae Jin Chae 3 , Natalia I Dmitrieva 9 , Mark McKenzie 1, 2 , Amanda Light 1 , Beverly K Barham 3 , Anne Jones 3 , Tina M Romeo 3 , Qing Zhou 3 , Ivona Aksentijevich 3 , James C Mullikin 14 , Andrew J Gross 15 , Anthony K Shum 16 , Edwin D Hawkins 1, 2 , Seth L Masters 1, 2 , Michael J Lenardo 12 , Manfred Boehm 9 , Sergio D Rosenzweig 8 , Manolis Pasparakis 10, 11 , Anne K Voss 1, 2 , Massimo Gadina 7 , Daniel L Kastner 3 , John Silke 1, 2
Affiliation
|
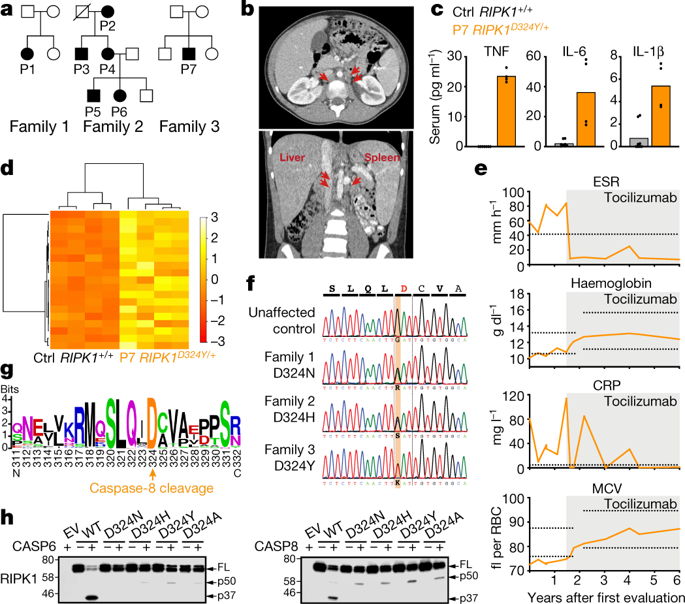
RIPK1 is a key regulator of innate immune signalling pathways. To ensure an optimal inflammatory response, RIPK1 is regulated post-translationally by well-characterized ubiquitylation and phosphorylation events, as well as by caspase-8-mediated cleavage 1 – 7 . The physiological relevance of this cleavage event remains unclear, although it is thought to inhibit activation of RIPK3 and necroptosis 8 . Here we show that the heterozygous missense mutations D324N, D324H and D324Y prevent caspase cleavage of RIPK1 in humans and result in an early-onset periodic fever syndrome and severe intermittent lymphadenopathy—a condition we term ‘cleavage-resistant RIPK1-induced autoinflammatory syndrome’. To define the mechanism for this disease, we generated a cleavage-resistant Ripk1 D325A mutant mouse strain. Whereas Ripk1 −/− mice died postnatally from systemic inflammation, Ripk1 D325A/D325A mice died during embryogenesis. Embryonic lethality was completely prevented by the combined loss of Casp8 and Ripk3 , but not by loss of Ripk3 or Mlkl alone. Loss of RIPK1 kinase activity also prevented Ripk1 D325A/D325A embryonic lethality, although the mice died before weaning from multi-organ inflammation in a RIPK3-dependent manner. Consistently, Ripk1 D325A/D325A and Ripk1 D325A /+ cells were hypersensitive to RIPK3-dependent TNF-induced apoptosis and necroptosis. Heterozygous Ripk1 D325A /+ mice were viable and grossly normal, but were hyper-responsive to inflammatory stimuli in vivo. Our results demonstrate the importance of caspase-mediated RIPK1 cleavage during embryonic development and show that caspase cleavage of RIPK1 not only inhibits necroptosis but also maintains inflammatory homeostasis throughout life. Heterozygous mutateons in the caspase-8 cleavage site of RIPK1 cause a range of autoinflammatory symptoms in humans, and caspase-8 cleavage of RIPK1 in a mouse model limits TNF-induced cell death and inflammation.
中文翻译:

阻止 RIPK1 的半胱天冬酶切割的突变导致自身炎症性疾病
RIPK1 是先天免疫信号通路的关键调节因子。为了确保最佳的炎症反应,RIPK1 在翻译后受到充分表征的泛素化和磷酸化事件以及 caspase-8 介导的切割 1-7 的调节。这种分裂事件的生理相关性仍不清楚,尽管它被认为可以抑制 RIPK3 的激活和坏死性凋亡 8。在这里,我们显示杂合错义突变 D324N、D324H 和 D324Y 阻止了人类中 RIPK1 的半胱天冬酶裂解,并导致早发性周期性发热综合征和严重的间歇性淋巴结病——我们将这种情况称为“抗裂解 RIPK1 诱导的自身炎症综合征”。为了确定这种疾病的机制,我们产生了一种抗切割的 Ripk1 D325A 突变小鼠品系。Ripk1 -/- 小鼠在出生后死于全身炎症,而 Ripk1 D325A/D325A 小鼠在胚胎发生期间死亡。Casp8 和 Ripk3 的联合丢失完全防止了胚胎致死性,但仅通过丢失 Ripk3 或 Mlk1 并不能完全防止胚胎致死。RIPK1 激酶活性的丧失也阻止了 Ripk1 D325A/D325A 胚胎致死率,尽管小鼠在以 RIPK3 依赖性方式从多器官炎症断奶前死亡。一致地,Ripk1 D325A/D325A 和 Ripk1 D325A /+ 细胞对 RIPK3 依赖性 TNF 诱导的细胞凋亡和坏死性凋亡高度敏感。杂合的 Ripk1 D325A /+ 小鼠存活且基本正常,但对体内的炎症刺激反应过度。我们的研究结果证明了 caspase 介导的 RIPK1 切割在胚胎发育过程中的重要性,并表明 RIPK1 的 caspase 切割不仅抑制坏死性凋亡,而且在整个生命过程中维持炎症稳态。RIPK1 的 caspase-8 切割位点中的杂合突变会导致人类出现一系列自身炎症症状,并且小鼠模型中 RIPK1 的 caspase-8 切割限制了 TNF 诱导的细胞死亡和炎症。
更新日期:2019-12-11
中文翻译:

阻止 RIPK1 的半胱天冬酶切割的突变导致自身炎症性疾病
RIPK1 是先天免疫信号通路的关键调节因子。为了确保最佳的炎症反应,RIPK1 在翻译后受到充分表征的泛素化和磷酸化事件以及 caspase-8 介导的切割 1-7 的调节。这种分裂事件的生理相关性仍不清楚,尽管它被认为可以抑制 RIPK3 的激活和坏死性凋亡 8。在这里,我们显示杂合错义突变 D324N、D324H 和 D324Y 阻止了人类中 RIPK1 的半胱天冬酶裂解,并导致早发性周期性发热综合征和严重的间歇性淋巴结病——我们将这种情况称为“抗裂解 RIPK1 诱导的自身炎症综合征”。为了确定这种疾病的机制,我们产生了一种抗切割的 Ripk1 D325A 突变小鼠品系。Ripk1 -/- 小鼠在出生后死于全身炎症,而 Ripk1 D325A/D325A 小鼠在胚胎发生期间死亡。Casp8 和 Ripk3 的联合丢失完全防止了胚胎致死性,但仅通过丢失 Ripk3 或 Mlk1 并不能完全防止胚胎致死。RIPK1 激酶活性的丧失也阻止了 Ripk1 D325A/D325A 胚胎致死率,尽管小鼠在以 RIPK3 依赖性方式从多器官炎症断奶前死亡。一致地,Ripk1 D325A/D325A 和 Ripk1 D325A /+ 细胞对 RIPK3 依赖性 TNF 诱导的细胞凋亡和坏死性凋亡高度敏感。杂合的 Ripk1 D325A /+ 小鼠存活且基本正常,但对体内的炎症刺激反应过度。我们的研究结果证明了 caspase 介导的 RIPK1 切割在胚胎发育过程中的重要性,并表明 RIPK1 的 caspase 切割不仅抑制坏死性凋亡,而且在整个生命过程中维持炎症稳态。RIPK1 的 caspase-8 切割位点中的杂合突变会导致人类出现一系列自身炎症症状,并且小鼠模型中 RIPK1 的 caspase-8 切割限制了 TNF 诱导的细胞死亡和炎症。

































 京公网安备 11010802027423号
京公网安备 11010802027423号