Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1
Nature ( IF 50.5 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41586-019-1830-y
Panfeng Tao 1 , Jinqiao Sun 2 , Zheming Wu 3 , Shihao Wang 1 , Jun Wang 1 , Wanjin Li 4 , Heling Pan 3 , Renkui Bai 5 , Jiahui Zhang 1 , Ying Wang 2 , Pui Y Lee 6 , Wenjing Ying 2 , Qinhua Zhou 2 , Jia Hou 2 , Wenjie Wang 2 , Bijun Sun 2 , Mi Yang 2 , Danru Liu 2 , Ran Fang 1 , Huan Han 1 , Zhaohui Yang 1 , Xin Huang 3 , Haibo Li 7 , Natalie Deuitch 8 , Yuan Zhang 9 , Dilan Dissanayake 10 , Katrina Haude 5 , Kirsty McWalter 5 , Chelsea Roadhouse 11 , Jennifer J MacKenzie 11, 12 , Ronald M Laxer 13 , Ivona Aksentijevich 14 , Xiaomin Yu 1 , Xiaochuan Wang 2 , Junying Yuan 4 , Qing Zhou 1, 15
Nature ( IF 50.5 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41586-019-1830-y
Panfeng Tao 1 , Jinqiao Sun 2 , Zheming Wu 3 , Shihao Wang 1 , Jun Wang 1 , Wanjin Li 4 , Heling Pan 3 , Renkui Bai 5 , Jiahui Zhang 1 , Ying Wang 2 , Pui Y Lee 6 , Wenjing Ying 2 , Qinhua Zhou 2 , Jia Hou 2 , Wenjie Wang 2 , Bijun Sun 2 , Mi Yang 2 , Danru Liu 2 , Ran Fang 1 , Huan Han 1 , Zhaohui Yang 1 , Xin Huang 3 , Haibo Li 7 , Natalie Deuitch 8 , Yuan Zhang 9 , Dilan Dissanayake 10 , Katrina Haude 5 , Kirsty McWalter 5 , Chelsea Roadhouse 11 , Jennifer J MacKenzie 11, 12 , Ronald M Laxer 13 , Ivona Aksentijevich 14 , Xiaomin Yu 1 , Xiaochuan Wang 2 , Junying Yuan 4 , Qing Zhou 1, 15
Affiliation
|
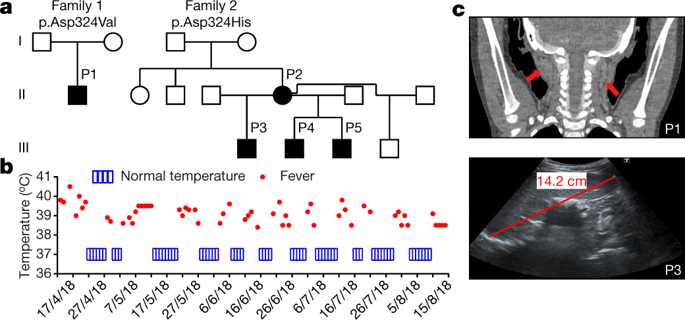
Activation of RIPK1 controls TNF-mediated apoptosis, necroptosis and inflammatory pathways 1 . Cleavage of human and mouse RIPK1 after residues D324 and D325, respectively, by caspase-8 separates the RIPK1 kinase domain from the intermediate and death domains. The D325A mutation in mouse RIPK1 leads to embryonic lethality during mouse development 2 , 3 . However, the functional importance of blocking caspase-8-mediated cleavage of RIPK1 on RIPK1 activation in humans is unknown. Here we identify two families with variants in RIPK1 (D324V and D324H) that lead to distinct symptoms of recurrent fevers and lymphadenopathy in an autosomal-dominant manner. Impaired cleavage of RIPK1 D324 variants by caspase-8 sensitized patients’ peripheral blood mononuclear cells to RIPK1 activation, apoptosis and necroptosis induced by TNF. The patients showed strong RIPK1-dependent activation of inflammatory signalling pathways and overproduction of inflammatory cytokines and chemokines compared with unaffected controls. Furthermore, we show that expression of the RIPK1 mutants D325V or D325H in mouse embryonic fibroblasts confers not only increased sensitivity to RIPK1 activation-mediated apoptosis and necroptosis, but also induction of pro-inflammatory cytokines such as IL-6 and TNF. By contrast, patient-derived fibroblasts showed reduced expression of RIPK1 and downregulated production of reactive oxygen species, resulting in resistance to necroptosis and ferroptosis. Together, these data suggest that human non-cleavable RIPK1 variants promote activation of RIPK1, and lead to an autoinflammatory disease characterized by hypersensitivity to apoptosis and necroptosis and increased inflammatory response in peripheral blood mononuclear cells, as well as a compensatory mechanism to protect against several pro-death stimuli in fibroblasts. A dominantly inherited human autoinflammatory disease caused by mutations in RIPK1 is identified, and RIPK1 mutations that prevent caspase-8 cleavage sensitize cells to apoptosis, necroptosis and inflammation.
中文翻译:

由 RIPK1 的不可切割变体引起的显性自身炎症性疾病
RIPK1 的激活控制 TNF 介导的细胞凋亡、坏死性凋亡和炎症通路 1。caspase-8 在残基 D324 和 D325 后分别切割人和小鼠 RIPK1,将 RIPK1 激酶域与中间域和死亡域分开。小鼠 RIPK1 中的 D325A 突变导致小鼠发育过程中的胚胎致死 2, 3 。然而,阻断 caspase-8 介导的 RIPK1 裂解对人类 RIPK1 激活的功能重要性尚不清楚。在这里,我们确定了两个具有 RIPK1 变异的家族(D324V 和 D324H),它们以常染色体显性方式导致反复发烧和淋巴结病的不同症状。caspase-8 对 RIPK1 D324 变体的切割受损使患者的外周血单核细胞对 TNF 诱导的 RIPK1 激活、细胞凋亡和坏死性凋亡敏感。与未受影响的对照相比,这些患者表现出强烈的 RIPK1 依赖性炎症信号通路激活和炎症细胞因子和趋化因子的过度产生。此外,我们表明 RIPK1 突变体 D325V 或 D325H 在小鼠胚胎成纤维细胞中的表达不仅增加了对 RIPK1 激活介导的细胞凋亡和坏死性凋亡的敏感性,而且还诱导了促炎细胞因子,如 IL-6 和 TNF。相比之下,患者来源的成纤维细胞表现出 RIPK1 的表达减少和活性氧的产生下调,导致对坏死性凋亡和铁死亡的抵抗。总之,这些数据表明人类不可切割的 RIPK1 变体促进了 RIPK1 的激活,并导致自身炎症性疾病,其特征是对细胞凋亡和坏死性凋亡的超敏反应以及外周血单核细胞的炎症反应增加,以及保护成纤维细胞免受几种促死亡刺激的补偿机制。确定了一种由 RIPK1 突变引起的显性遗传的人类自身炎症性疾病,防止 caspase-8 切割的 RIPK1 突变使细胞对细胞凋亡、坏死性凋亡和炎症敏感。
更新日期:2019-12-11
中文翻译:

由 RIPK1 的不可切割变体引起的显性自身炎症性疾病
RIPK1 的激活控制 TNF 介导的细胞凋亡、坏死性凋亡和炎症通路 1。caspase-8 在残基 D324 和 D325 后分别切割人和小鼠 RIPK1,将 RIPK1 激酶域与中间域和死亡域分开。小鼠 RIPK1 中的 D325A 突变导致小鼠发育过程中的胚胎致死 2, 3 。然而,阻断 caspase-8 介导的 RIPK1 裂解对人类 RIPK1 激活的功能重要性尚不清楚。在这里,我们确定了两个具有 RIPK1 变异的家族(D324V 和 D324H),它们以常染色体显性方式导致反复发烧和淋巴结病的不同症状。caspase-8 对 RIPK1 D324 变体的切割受损使患者的外周血单核细胞对 TNF 诱导的 RIPK1 激活、细胞凋亡和坏死性凋亡敏感。与未受影响的对照相比,这些患者表现出强烈的 RIPK1 依赖性炎症信号通路激活和炎症细胞因子和趋化因子的过度产生。此外,我们表明 RIPK1 突变体 D325V 或 D325H 在小鼠胚胎成纤维细胞中的表达不仅增加了对 RIPK1 激活介导的细胞凋亡和坏死性凋亡的敏感性,而且还诱导了促炎细胞因子,如 IL-6 和 TNF。相比之下,患者来源的成纤维细胞表现出 RIPK1 的表达减少和活性氧的产生下调,导致对坏死性凋亡和铁死亡的抵抗。总之,这些数据表明人类不可切割的 RIPK1 变体促进了 RIPK1 的激活,并导致自身炎症性疾病,其特征是对细胞凋亡和坏死性凋亡的超敏反应以及外周血单核细胞的炎症反应增加,以及保护成纤维细胞免受几种促死亡刺激的补偿机制。确定了一种由 RIPK1 突变引起的显性遗传的人类自身炎症性疾病,防止 caspase-8 切割的 RIPK1 突变使细胞对细胞凋亡、坏死性凋亡和炎症敏感。































 京公网安备 11010802027423号
京公网安备 11010802027423号