当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Regulatory Cysteine Residue Mediates Reversible Inactivation of NAD+-Dependent Aldehyde Dehydrogenases to Promote Oxidative Stress Response.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-12-16 , DOI: 10.1021/acschembio.9b00662 Yugang Zhang 1 , Miao Wang 1 , Hening Lin 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-12-16 , DOI: 10.1021/acschembio.9b00662 Yugang Zhang 1 , Miao Wang 1 , Hening Lin 2
Affiliation
|
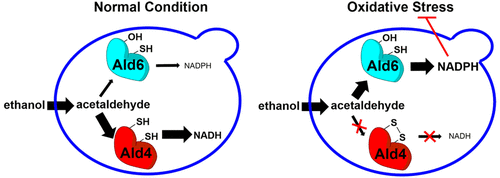
Aldehyde dehydrogenases (ALDHs) are a large family of enzymes that oxidize aldehydes into carboxylic acids. All ALDHs have a conserved catalytic cysteine residue but different cofactor preferences for NAD+ or NADP+. We discovered a CC motif composed of the catalytic and an adjacent cysteine, which are prone to disulfide bond formation under oxidative stress. This facilitates rapid detection of and response to oxidants, as well as protects the catalytic cysteine from overoxidation into irreversible products. In ALDHs, the CC motif only exists in NAD+-dependent ones, which leads to selective inhibition of NAD+-dependent ALDHs under oxidative stress, diverting carbon sources to the NADPH producing ALDHs. This alleviates the oxidative stress and promotes cell survival. Our findings revealed a novel regulatory mechanism for ALDHs that functions in the oxidative stress response. Many enzymes with catalytic cysteine residues have proximal cysteine, suggesting that such a regulatory mechanism may be general.
中文翻译:

监管的半胱氨酸残基介导NAD +依赖醛脱氢酶的可逆失活,以促进氧化应激反应。
醛脱氢酶(ALDHs)是将醛氧化为羧酸的一大类酶。所有ALDHs均具有保守的催化半胱氨酸残基,但对于NAD +或NADP +的辅因子偏好不同。我们发现了由催化和相邻的半胱氨酸组成的CC基序,它们在氧化应激下易于形成二硫键。这有利于氧化剂的快速检测和响应,并保护催化性半胱氨酸免于过度氧化成不可逆产物。在ALDHs中,CC基序仅存在于NAD +依赖性ALDH中,这导致在氧化应激下选择性抑制NAD +依赖性ALDHs,将碳源转移至产生NADPH的ALDHs。这减轻了氧化应激并促进了细胞存活。我们的发现揭示了在氧化应激反应中起作用的ALDHs的新型调节机制。许多具有催化性半胱氨酸残基的酶都具有近端半胱氨酸,这表明这种调节机制可能是普遍的。
更新日期:2019-12-17
中文翻译:

监管的半胱氨酸残基介导NAD +依赖醛脱氢酶的可逆失活,以促进氧化应激反应。
醛脱氢酶(ALDHs)是将醛氧化为羧酸的一大类酶。所有ALDHs均具有保守的催化半胱氨酸残基,但对于NAD +或NADP +的辅因子偏好不同。我们发现了由催化和相邻的半胱氨酸组成的CC基序,它们在氧化应激下易于形成二硫键。这有利于氧化剂的快速检测和响应,并保护催化性半胱氨酸免于过度氧化成不可逆产物。在ALDHs中,CC基序仅存在于NAD +依赖性ALDH中,这导致在氧化应激下选择性抑制NAD +依赖性ALDHs,将碳源转移至产生NADPH的ALDHs。这减轻了氧化应激并促进了细胞存活。我们的发现揭示了在氧化应激反应中起作用的ALDHs的新型调节机制。许多具有催化性半胱氨酸残基的酶都具有近端半胱氨酸,这表明这种调节机制可能是普遍的。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号