当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Solandelactone I
Journal of Natural Products ( IF 3.3 ) Pub Date : 2015-11-12 00:00:00 , DOI: 10.1021/acs.jnatprod.5b00757 Nils C. Eichenauer 1 , Roxanne Tschersich 1 , Jörg Pietruszka 1, 2
Journal of Natural Products ( IF 3.3 ) Pub Date : 2015-11-12 00:00:00 , DOI: 10.1021/acs.jnatprod.5b00757 Nils C. Eichenauer 1 , Roxanne Tschersich 1 , Jörg Pietruszka 1, 2
Affiliation
|
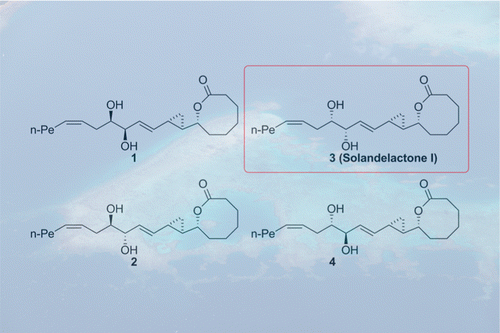
Since the marine natural products solandelactones A–I were isolated from the hydroid Solanderia secunda and investigated by Seo et al. in 1996, considerable synthetic efforts toward these marine oxylipins followed. However, the structure elucidation of solandelactone I remained incomplete, and no synthesis has been reported. On the basis of our retrosynthetic analysis, the key building blocks were combined in a Horner–Wadsworth–Emmons reaction to create two common intermediates for the stereodivergent synthesis of all four diastereomers 1–4 matching the proposed structure of solandelactone I. Comparison of the published analytical data of natural product solandelactone I and data obtained from the synthetic endeavor toward diastereomers 1–4 enabled the structure assignment of isomer 3; the proposed biosynthetic pathway for marine oxylipins also supports the result.
中文翻译:

Solandelactone I的全合成
由于海洋天然产物solandelactones A-I是从分离水螅Solanderia塞康达和由Seo等人调查。在1996年,人们对这些海洋上的脂蛋白进行了大量的合成研究。然而,苏糖内酯I的结构解析仍然不完全,并且没有合成的报道。在我们的逆合成分析的基础上,所述键积木合并在Horner-Wadsworth-Emmons反应,为所有四个非对映体的合成stereodivergent创建两个共同中间体1 - 4匹配solandelactone I.公布的比较的所提出的结构天然产物solandelactone I的分析数据以及从合成方法中获得的非对映异构体数据1– 4启用了异构体3的结构分配;拟议的海洋氧化脂生物合成途径也支持该结果。
更新日期:2015-11-12
中文翻译:

Solandelactone I的全合成
由于海洋天然产物solandelactones A-I是从分离水螅Solanderia塞康达和由Seo等人调查。在1996年,人们对这些海洋上的脂蛋白进行了大量的合成研究。然而,苏糖内酯I的结构解析仍然不完全,并且没有合成的报道。在我们的逆合成分析的基础上,所述键积木合并在Horner-Wadsworth-Emmons反应,为所有四个非对映体的合成stereodivergent创建两个共同中间体1 - 4匹配solandelactone I.公布的比较的所提出的结构天然产物solandelactone I的分析数据以及从合成方法中获得的非对映异构体数据1– 4启用了异构体3的结构分配;拟议的海洋氧化脂生物合成途径也支持该结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号