Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2019-12-06 , DOI: 10.1016/j.jorganchem.2019.121068 Alexander S. Antonov , Victor G. Bardakov , Valeriia V. Mulloyarova
|
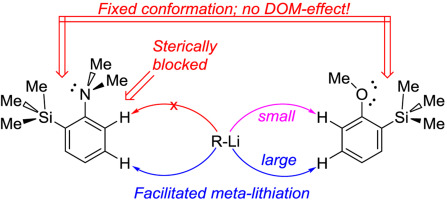
The influence of the bulky trimethylsilyl substituent on the selectivity of metallation of dimethylaniline, anisole and 1-dimethylaminonaphthalene is studied. The neighboring SiMe3 group forces dimethylamino and methoxy groups to occupy a conformation with an unshared electron pair turned towards silicon atom. This forced conformation prevents NMe2 and OMe groups from providing the DOM-effect, thus facilitating meta-metallation with the use of bulky LiCKOR. While the inverted NMe2 group completely suppresses ortho-metallation, the less bulky and more electron withdrawing OMe group demonstrates more rotation freedom allowing selective ortho-metallation with smaller reagents such as n-BuLi or tert-BuLi.
中文翻译:

在空间上促进了含给电子基团的芳烃的元锂化反应
研究了庞大的三甲基甲硅烷基取代基对二甲基苯胺,苯甲醚和1-二甲基氨基萘金属化选择性的影响。相邻的SiMe 3基团迫使二甲基氨基和甲氧基占据一个构象,其中未共享的电子对转向硅原子。这迫使构象防止NME 2和OME组从提供所述DOM-效果,从而有利于元与使用笨重LiCKOR的-metallation。而反向的NME 2组完全抑制邻-metallation中,体积更小且多个吸电子基团OME演示多个旋转自由度,允许选择性邻-metallation具有较小试剂如n -BuLi或叔-BuLi。































 京公网安备 11010802027423号
京公网安备 11010802027423号