当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and evaluation of 4-(1,3,4-oxadiazol-2-yl)-benzenesulfonamides as potent carbonic anhydrase inhibitors.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.bmcl.2019.126874 Chaofu Yang 1 , Yan Feng 2 , Xu Yang 3 , Mingxia Sun 4 , Zhenwang Li 5 , Xuan Liu 3 , Liang Lu 3 , Xianyu Sun 5 , Jiwen Zhang 4 , Xinhua He 3
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.bmcl.2019.126874 Chaofu Yang 1 , Yan Feng 2 , Xu Yang 3 , Mingxia Sun 4 , Zhenwang Li 5 , Xuan Liu 3 , Liang Lu 3 , Xianyu Sun 5 , Jiwen Zhang 4 , Xinhua He 3
Affiliation
|
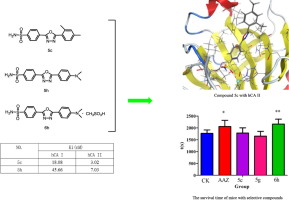
Human Carbonic anhydrase (hCA) I and II are crucial targets for anti-acute mountain sickness. Twenty-one 4-(1,3,4-oxadiazol-2-yl) benzenesulfonamides were synthesized and screened against these two isoforms. The results illustrated that 5c, 5g, 5h, 5k were more potent against both hCA I and II than clinical drug AAZ. In particular, the value of compound 5c with hCA I (18.08 nM) was over 84-fold more than of AAZ with hCA I. The data of docking simulations were also in accord with the tendency of inhibitive activities. Furthermore, compound 6h, the methanesulfonate of 5h, showed better anti-hypoxia activity than AAZ in vivo, making it interesting lead compound.
中文翻译:

4-(1,3,4-恶二唑-2-基)-苯磺酰胺作为有效碳酸酐酶抑制剂的合成和评价。
人碳酸酐酶 (hCA) I 和 II 是抗急性高山病的重要靶点。合成了 21 种 4-(1,3,4-恶二唑-2-基)苯磺酰胺,并针对这两种异构体进行了筛选。结果表明,5c、5g、5h、5k 比临床药物 AAZ 更有效地对抗 hCA I 和 II。特别是,化合物5c与hCA I的值(18.08 nM)是AAZ与hCA I的值的84倍以上。对接模拟的数据也与抑制活性的趋势一致。此外,化合物6h(5h的甲磺酸盐)在体内表现出比AAZ更好的抗缺氧活性,使其成为令人感兴趣的先导化合物。
更新日期:2019-12-05
中文翻译:

4-(1,3,4-恶二唑-2-基)-苯磺酰胺作为有效碳酸酐酶抑制剂的合成和评价。
人碳酸酐酶 (hCA) I 和 II 是抗急性高山病的重要靶点。合成了 21 种 4-(1,3,4-恶二唑-2-基)苯磺酰胺,并针对这两种异构体进行了筛选。结果表明,5c、5g、5h、5k 比临床药物 AAZ 更有效地对抗 hCA I 和 II。特别是,化合物5c与hCA I的值(18.08 nM)是AAZ与hCA I的值的84倍以上。对接模拟的数据也与抑制活性的趋势一致。此外,化合物6h(5h的甲磺酸盐)在体内表现出比AAZ更好的抗缺氧活性,使其成为令人感兴趣的先导化合物。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号