Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.cej.2019.123694 Jie Xu , Fengtao Yu , Jianli Hua , Weiqiang Tang , Chao Yang , Shuozhen Hu , Shuangliang Zhao , Xinsheng Zhang , Zhong Xin , Dongfang Niu
|
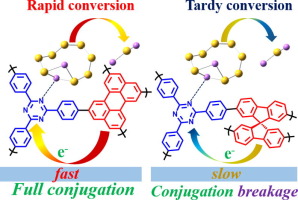
Triazine-based framework is an attractive organic polymer with microporous structure and high nitrogen content that could retard the shuttle of polysulfides for high-performance lithium-sulfur (Li-S) batteries. However, insight into the role of π-conjugated donor of triazine-based polymer in immobilizing and catalytzing polysulfides is still deficient. Here, two homologous conjugated buildings of triazine unit combining with perylene (CTP-1) and spirobifluorene (CTP-2) were reasonably synthesized through one-pot Suzuki-Miyaura coupling reaction with a high yield of 92% and served as the polysulfides immobilizer and catalyst to achieve high-performance Li-S batteries. Compared with the counterpart of CTP-2, the fully conjugated CTP-1 modified separator possesses a higher ionic conductivity and Li+ transference number presenting more efficient shuttle inhibition and faster kinetics in catalyzing polysulfides conversion. Theoretical calculations demonstrate that the CTP-1 with perylene donor features narrower bandgap and faster electron transfer exhibiting stronger binding ability toward polysulfides than CTP-2. This work broadens the understanding of donor dominated chemical immobilizing and catalysis of triazine-based materials in Li-S batteries, and provides guidance for the future design of organic materials in other energy storage systems.
中文翻译:

施主为主的三嗪基微孔聚合物作为高性能硫化锂电池的多硫化物固定剂和催化剂
基于三嗪的骨架是一种有吸引力的有机聚合物,具有微孔结构和高氮含量,可以阻止多硫化物对高性能锂硫(Li-S)电池的穿梭。然而,对于基于三嗪的聚合物的π共轭供体在固定和催化多硫化物方面的作用的认识仍然不足。在此,通过一锅Suzuki-Miyaura偶联反应以92%的高收率合理合成了三嗪单元与per(CTP-1)和螺二芴(CTP-2)结合的两个同源共轭结构,并用作多硫化物固定剂和催化剂,以实现高性能的Li-S电池。与CTP-2相比,完全共轭的CTP-1改性隔膜具有更高的离子电导率和Li +转移数在催化多硫化物转化中表现出更有效的穿梭抑制和更快的动力学。理论计算表明,具有per供体的CTP-1具有较窄的带隙和较快的电子传递,与CTP-2相比,对聚硫化物的结合能力更强。这项工作拓宽了Li-S电池中供体为主的化学固定化和三嗪基材料催化的理解,并为其他储能系统中有机材料的未来设计提供了指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号