Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Leishmania aethiopica cell-to-cell spreading involves caspase-3, AkT, and NF-κB but not PKC-δ activation and involves uptake of LAMP-1-positive bodies containing parasites.
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-12-20 , DOI: 10.1111/febs.15166 Medhavi Ranatunga 1 , Rajeev Rai 1 , Simon C W Richardson 1 , Paul Dyer 1 , Laurence Harbige 1 , Andrew Deacon 1 , Lauren Pecorino 1 , Giulia T M Getti 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-12-20 , DOI: 10.1111/febs.15166 Medhavi Ranatunga 1 , Rajeev Rai 1 , Simon C W Richardson 1 , Paul Dyer 1 , Laurence Harbige 1 , Andrew Deacon 1 , Lauren Pecorino 1 , Giulia T M Getti 1
Affiliation
|
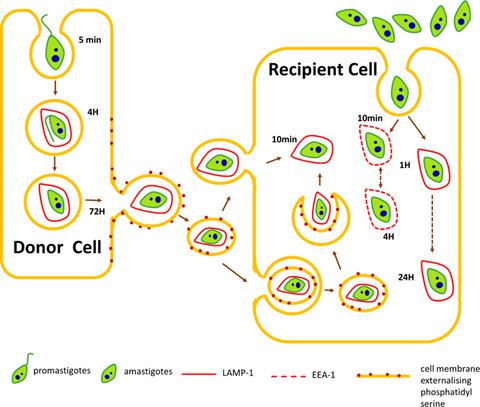
Development of human leishmaniasis is dependent on the ability of intracellular Leishmania parasites to spread and enter macrophages. The mechanism through which free promastigotes and amastigotes bind and enter host macrophages has been previously investigated; however, little is known about intracellular trafficking and cell-to-cell spreading. In this study, the mechanism involved in the spreading of Leishmania aethiopica and Leishmania mexicana was investigated. A significant increase in phosphatidylserine (PS) exhibition, cytochrome C release, and active caspase-3 expression was detected (P < 0.05) during L. aethiopica, but not L. mexicana spreading. A decrease (P < 0.05) of protein kinase B (Akt) protein and BCL2-associated agonist of cell death (BAD) phosphorylation was also observed. The nuclear factor kappa-light-chain enhancer of activated B cells (NF-kB) signaling pathway and pro-apoptotic protein protein kinase C delta (PKC-δ) were downregulated while inhibition of caspase-3 activation prevented L. aethiopica spreading. Overall suggesting that L. aethiopica induces host cell's apoptosis during spreading in a caspase-3-dependent manner. The trafficking of amastigotes within macrophages following cell-to-cell spreading differed from that of axenic parasites and involved co-localization with lysosomal-associated membrane protein 1 (LAMP-1) within 10 min postinfection. Interestingly, following infection with axenic amastigotes and promastigotes, co-localization of parasites with LAMP-1-positive structures took place at 1 and 4 h, respectively, suggesting that the membrane coat and LAMP-1 protein were derived from the donor cell. Collectively, these findings indicate that host cell apoptosis, demonstrated by PS exhibition, caspase-3 activation, cytochrome C release, downregulation of Akt, BAD phosphorylation, NF-kB activation, and independent of PKC-δ expression, is involved in L. aethiopica spreading. Moreover, L. aethiopica parasites associate with LAMP-rich structures when taken up by neighboring macrophages.
中文翻译:

埃塞俄比亚利什曼原虫细胞间传播涉及 caspase-3、AkT 和 NF-κB,但不涉及 PKC-δ 激活,并涉及摄取含有寄生虫的 LAMP-1 阳性体。
人类利什曼病的发展取决于细胞内利什曼原虫传播和进入巨噬细胞的能力。先前已研究过游离前鞭毛体和无鞭毛体结合并进入宿主巨噬细胞的机制。然而,人们对细胞内运输和细胞间传播知之甚少。在这项研究中,研究了埃塞俄比亚利什曼原虫和墨西哥利什曼原虫传播的机制。在埃塞俄比亚乳杆菌传播期间,检测到磷脂酰丝氨酸 (PS) 表达、细胞色素 C 释放和活性 caspase-3 表达显着增加 (P < 0.05),但在墨西哥乳杆菌传播期间未检测到。还观察到蛋白激酶 B (Akt) 蛋白和 BCL2 相关细胞死亡激动剂 (BAD) 磷酸化降低 (P < 0.05)。活化 B 细胞核因子 kappa-轻链增强子 (NF-kB) 信号通路和促凋亡蛋白激酶 C δ (PKC-δ) 下调,同时抑制 caspase-3 激活可阻止埃塞俄比亚乳杆菌扩散。总体而言,埃塞俄比亚乳杆菌在传播过程中以 caspase-3 依赖性方式诱导宿主细胞凋亡。细胞间传播后巨噬细胞内无鞭毛体的运输与纯寄生虫不同,并且涉及感染后 10 分钟内与溶酶体相关膜蛋白 1 (LAMP-1) 的共定位。有趣的是,在用纯无鞭毛体和前鞭毛体感染后,寄生虫与 LAMP-1 阳性结构的共定位分别发生在 1 小时和 4 小时,这表明膜涂层和 LAMP-1 蛋白源自供体细胞。 总的来说,这些发现表明,埃塞俄比亚乳杆菌中涉及宿主细胞凋亡(通过 PS 展示、caspase-3 激活、细胞色素 C 释放、Akt 下调、BAD 磷酸化、NF-kB 激活以及独立于 PKC-δ 表达来证明)传播。此外,当被邻近的巨噬细胞吞噬时,埃塞俄比亚乳杆菌寄生虫会与富含 LAMP 的结构结合。
更新日期:2019-12-20
中文翻译:

埃塞俄比亚利什曼原虫细胞间传播涉及 caspase-3、AkT 和 NF-κB,但不涉及 PKC-δ 激活,并涉及摄取含有寄生虫的 LAMP-1 阳性体。
人类利什曼病的发展取决于细胞内利什曼原虫传播和进入巨噬细胞的能力。先前已研究过游离前鞭毛体和无鞭毛体结合并进入宿主巨噬细胞的机制。然而,人们对细胞内运输和细胞间传播知之甚少。在这项研究中,研究了埃塞俄比亚利什曼原虫和墨西哥利什曼原虫传播的机制。在埃塞俄比亚乳杆菌传播期间,检测到磷脂酰丝氨酸 (PS) 表达、细胞色素 C 释放和活性 caspase-3 表达显着增加 (P < 0.05),但在墨西哥乳杆菌传播期间未检测到。还观察到蛋白激酶 B (Akt) 蛋白和 BCL2 相关细胞死亡激动剂 (BAD) 磷酸化降低 (P < 0.05)。活化 B 细胞核因子 kappa-轻链增强子 (NF-kB) 信号通路和促凋亡蛋白激酶 C δ (PKC-δ) 下调,同时抑制 caspase-3 激活可阻止埃塞俄比亚乳杆菌扩散。总体而言,埃塞俄比亚乳杆菌在传播过程中以 caspase-3 依赖性方式诱导宿主细胞凋亡。细胞间传播后巨噬细胞内无鞭毛体的运输与纯寄生虫不同,并且涉及感染后 10 分钟内与溶酶体相关膜蛋白 1 (LAMP-1) 的共定位。有趣的是,在用纯无鞭毛体和前鞭毛体感染后,寄生虫与 LAMP-1 阳性结构的共定位分别发生在 1 小时和 4 小时,这表明膜涂层和 LAMP-1 蛋白源自供体细胞。 总的来说,这些发现表明,埃塞俄比亚乳杆菌中涉及宿主细胞凋亡(通过 PS 展示、caspase-3 激活、细胞色素 C 释放、Akt 下调、BAD 磷酸化、NF-kB 激活以及独立于 PKC-δ 表达来证明)传播。此外,当被邻近的巨噬细胞吞噬时,埃塞俄比亚乳杆菌寄生虫会与富含 LAMP 的结构结合。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号