当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Casein kinase-1γ1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41419-019-2146-4 Song-Yi Lee 1 , Hyunjoo Kim 1 , Cathena Meiling Li 1 , Jaemin Kang 1 , Ayaz Najafov 2 , Muhah Jung 1 , Soosung Kang 3 , Shaomeng Wang 4 , Junying Yuan 2 , Yong-Keun Jung 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41419-019-2146-4 Song-Yi Lee 1 , Hyunjoo Kim 1 , Cathena Meiling Li 1 , Jaemin Kang 1 , Ayaz Najafov 2 , Muhah Jung 1 , Soosung Kang 3 , Shaomeng Wang 4 , Junying Yuan 2 , Yong-Keun Jung 1
Affiliation
|
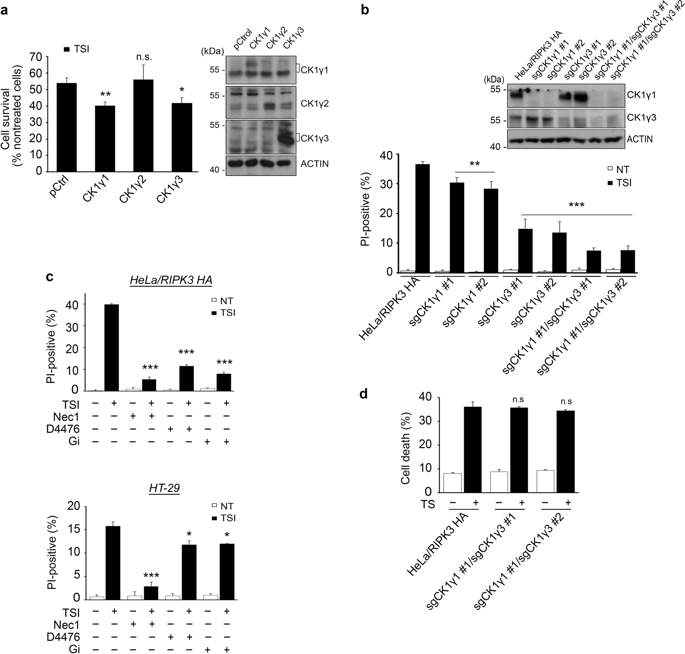
Upon necroptosis activation, receptor interacting serine/threonine kinase (RIPK)1 and RIPK3 form a necrosome complex with pseudokinase mixed lineage kinase-like (MLKL). Although protein phosphorylation is a key event for RIPK1 and RIPK3 activation in response to a necroptosis signal, relatively little is known about other factors that might regulate the activity of these kinases or necrosome formation. Through a gain-of-function screen with 546 kinases and 127 phosphatases, we identified casein kinase 1 gamma (CK1γ) as a candidate necroptosis-promoting factor. Here, we show that the decreased activity or amounts of CK1γ1 and CK1γ3, either by treatment with a chemical inhibitor or knockdown in cells, reduced TNFα-induced necroptosis. Conversely, ectopic expression of CK1γ1 or CK1γ3 exacerbated necroptosis, but not apoptosis. Similar to RIPK1 and RIPK3, CK1γ1 was also cleaved at Asp343 by caspase-8 during apoptosis. CK1γ1 and CK1γ3 formed a protein complex and were recruited to the necrosome harboring RIPK1, RIPK3 and MLKL. In particular, an autophosphorylated form of CK1γ3 at Ser344/345 was detected in the necrosome and was required to mediate the necroptosis. In addition, in vitro assays with purified proteins showed that CK1γ phosphorylated RIPK3, affecting its activity, and in vivo assays showed that the CK1γ-specific inhibitor Gi prevented abrupt death in mice with hypothermia in a model of TNFα-induced systemic inflammatory response syndrome. Collectively, these data suggest that CK1γ1 and CK1γ3 are required for TNFα-induced necroptosis likely by regulating RIPK3.
中文翻译:

酪蛋白激酶1γ1和3通过RIPK3刺激肿瘤坏死因子诱导的坏死性肾病。
坏死病激活后,受体相互作用的丝氨酸/苏氨酸激酶(RIPK)1和RIPK3与假激酶混合谱系激酶样(MLKL)形成坏死复合物。尽管蛋白质磷酸化是响应坏死病信号而激活RIPK1和RIPK3的关键事件,但对其他可能调控这些激酶或坏死体活性的因素知之甚少。通过具有546个激酶和127个磷酸酶的功能增益筛选,我们确定了酪蛋白激酶1γ(CK1γ)作为候选的坏死病促进因子。在这里,我们表明,通过化学抑制剂处理或细胞内敲低,CK1γ1和CK1γ3的活性或含量降低,从而降低了TNFα诱导的坏死性肾病。相反,CK1γ1或CK1γ3的异位表达加剧了坏死性肾病,但没有引起细胞凋亡。与RIPK1和RIPK3相似,CK1γ1在凋亡过程中也被caspase-8在Asp343处裂解。CK1γ1和CK1γ3形成蛋白质复合物,并被募集到具有RIPK1,RIPK3和MLKL的坏死体中。特别地,在坏死体中检测到Ser344 / 345处的CK1γ3的自磷酸化形式,并且是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。CK1γ1和CK1γ3形成蛋白质复合物,并被募集到具有RIPK1,RIPK3和MLKL的坏死体中。特别地,在坏死体中检测到Ser344 / 345处的CK1γ3的自磷酸化形式,并且是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。CK1γ1和CK1γ3形成蛋白质复合物,并被募集到具有RIPK1,RIPK3和MLKL的坏死体中。特别地,在坏死体中检测到Ser344 / 345处的CK1γ3的自磷酸化形式,并且是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。在坏死体中检测到Ser344 / 345处CK1γ3的自磷酸化形式,是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。在坏死体中检测到Ser344 / 345处CK1γ3的自磷酸化形式,是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。
更新日期:2019-12-05
中文翻译:

酪蛋白激酶1γ1和3通过RIPK3刺激肿瘤坏死因子诱导的坏死性肾病。
坏死病激活后,受体相互作用的丝氨酸/苏氨酸激酶(RIPK)1和RIPK3与假激酶混合谱系激酶样(MLKL)形成坏死复合物。尽管蛋白质磷酸化是响应坏死病信号而激活RIPK1和RIPK3的关键事件,但对其他可能调控这些激酶或坏死体活性的因素知之甚少。通过具有546个激酶和127个磷酸酶的功能增益筛选,我们确定了酪蛋白激酶1γ(CK1γ)作为候选的坏死病促进因子。在这里,我们表明,通过化学抑制剂处理或细胞内敲低,CK1γ1和CK1γ3的活性或含量降低,从而降低了TNFα诱导的坏死性肾病。相反,CK1γ1或CK1γ3的异位表达加剧了坏死性肾病,但没有引起细胞凋亡。与RIPK1和RIPK3相似,CK1γ1在凋亡过程中也被caspase-8在Asp343处裂解。CK1γ1和CK1γ3形成蛋白质复合物,并被募集到具有RIPK1,RIPK3和MLKL的坏死体中。特别地,在坏死体中检测到Ser344 / 345处的CK1γ3的自磷酸化形式,并且是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。CK1γ1和CK1γ3形成蛋白质复合物,并被募集到具有RIPK1,RIPK3和MLKL的坏死体中。特别地,在坏死体中检测到Ser344 / 345处的CK1γ3的自磷酸化形式,并且是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。CK1γ1和CK1γ3形成蛋白质复合物,并被募集到具有RIPK1,RIPK3和MLKL的坏死体中。特别地,在坏死体中检测到Ser344 / 345处的CK1γ3的自磷酸化形式,并且是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。在坏死体中检测到Ser344 / 345处CK1γ3的自磷酸化形式,是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。在坏死体中检测到Ser344 / 345处CK1γ3的自磷酸化形式,是介导坏死性坏死所必需的。此外,纯化蛋白的体外测定表明CK1γ磷酸化的RIPK3会影响其活性,而体内测定表明CK1γ特异性抑制剂Gi在TNFα诱导的全身性炎症反应综合征模型中可防止体温过低小鼠的突然死亡。总体而言,这些数据表明,CK1γ1和CK1γ3是TNFα诱导的坏死性坏死所必需的,可能是通过调节RIPK3来实现的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号