当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon dioxide is tightly bound in the [Co(Pyridine)(CO2)]− anionic complex
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-11-13 14:48:10 , DOI: 10.1063/1.4935573
Jacob D. Graham 1 , Allyson M. Buytendyk 1 , Xinxing Zhang 1 , Seong K. Kim 2 , Kit H. Bowen 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-11-13 14:48:10 , DOI: 10.1063/1.4935573
Jacob D. Graham 1 , Allyson M. Buytendyk 1 , Xinxing Zhang 1 , Seong K. Kim 2 , Kit H. Bowen 1
Affiliation
|
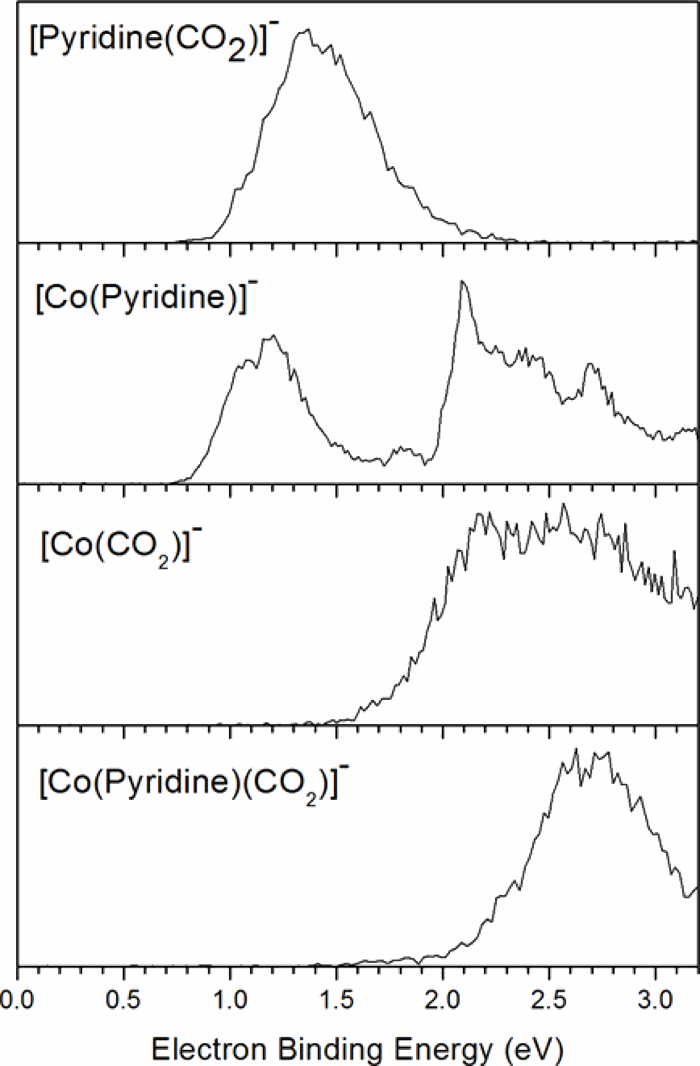
The [Co(Pyridine)(CO2)]− anionic complex was studied through the combination of photoelectron spectroscopy and density functional theory calculations. This complex was envisioned as a primitive model system for studying CO2 binding to negatively charged sites in metal organic frameworks. The vertical detachment energy (VDE) measured via the photoelectron spectrum is 2.7 eV. Our calculations imply a structure for [Co(Pyridine)(CO2)]− in which a central cobalt atom is bound to pyridine and CO2 moieties on either sides. This structure was validated by acceptable agreement between the calculated and measured VDE values. Based on our calculations, we found CO2 to be bound within the anionic complex by 1.4 eV.
中文翻译:

二氧化碳紧密结合在[Co(吡啶)(CO2)]-阴离子络合物中
通过结合光电子能谱和密度泛函理论计算研究了[Co(吡啶)(CO 2)] -阴离子络合物。该复合物被设想为一种原始模型系统,用于研究CO 2与金属有机骨架中带负电荷的位点的结合。经由光电子能谱测量的垂直脱离能(VDE)为2.7eV。我们的计算暗示了[Co(吡啶)(CO 2)] -的结构,其中中心钴原子与两侧的吡啶和CO 2部分键合。通过计算的和测量的VDE值之间可接受的一致性验证了此结构。根据我们的计算,我们发现了CO 2 在阴离子络合物中的结合力为1.4 eV。
更新日期:2015-11-14
中文翻译:

二氧化碳紧密结合在[Co(吡啶)(CO2)]-阴离子络合物中
通过结合光电子能谱和密度泛函理论计算研究了[Co(吡啶)(CO 2)] -阴离子络合物。该复合物被设想为一种原始模型系统,用于研究CO 2与金属有机骨架中带负电荷的位点的结合。经由光电子能谱测量的垂直脱离能(VDE)为2.7eV。我们的计算暗示了[Co(吡啶)(CO 2)] -的结构,其中中心钴原子与两侧的吡啶和CO 2部分键合。通过计算的和测量的VDE值之间可接受的一致性验证了此结构。根据我们的计算,我们发现了CO 2 在阴离子络合物中的结合力为1.4 eV。

































 京公网安备 11010802027423号
京公网安备 11010802027423号