当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereodivergent assembly of tetrahydro-γ-carbolines via synergistic catalytic asymmetric cascade reaction.
Nature Communications ( IF 14.7 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41467-019-13529-z Shi-Ming Xu 1 , Liang Wei 1 , Chong Shen 1 , Lu Xiao 1 , Hai-Yan Tao 1 , Chun-Jiang Wang 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41467-019-13529-z Shi-Ming Xu 1 , Liang Wei 1 , Chong Shen 1 , Lu Xiao 1 , Hai-Yan Tao 1 , Chun-Jiang Wang 1, 2
Affiliation
|
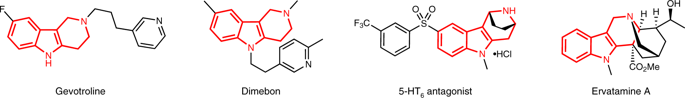
Enantiomerically enriched indole-containing heterocycles play a vital role in bioscience, medicine, and chemistry. As one of the most attractive subtypes of indole alkaloids, highly substituted tetrahydro-γ-carbolines are the basic structural unit in many natural products and pharmaceuticals. However, the syntheses of tetrahydro-γ-carbolines with high functionalities from readily available reagents are significant challenging. In particular, the stereodivergent syntheses of tetrahydro-γ-carbolines containing multi-stereogenic centers remain quite difficult. Herein, we report an expedient and stereodivergent assembly of tetrahydro-γ-carbolines with remarkably high levels of stereoselective control in an efficient cascade process from aldimine esters and indolyl allylic carbonates via a synergistic Cu/Ir catalyst system. Control experiments-guided optimization of synergistic catalysts and mechanistic investigations reveal that a stereodivergent allylation reaction and a subsequent highly stereoselective iso-Pictet-Spengler cyclization are the key elements to success.
中文翻译:

四氢-γ-咔啉通过催化催化不对称级联反应的立体发散组装。
对映体富集的含吲哚的杂环在生物科学,医学和化学中起着至关重要的作用。作为吲哚生物碱最吸引人的亚型之一,高度取代的四氢-γ-咔啉是许多天然产物和药物中的基本结构单元。然而,由容易获得的试剂合成具有高官能度的四氢-γ-咔啉是巨大的挑战。尤其是,含有多立体中心的四氢-γ-咔啉的立体发散性合成仍然非常困难。在本文中,我们报告了在高效的级联过程中,通过协同Cu / Ir催化剂体系从醛亚胺酯和吲哚基烯丙基碳酸酯得到的具有立体异构控制显着高水平的四氢-γ-咔啉的便利且立体发散的组装体。
更新日期:2019-12-05
中文翻译:

四氢-γ-咔啉通过催化催化不对称级联反应的立体发散组装。
对映体富集的含吲哚的杂环在生物科学,医学和化学中起着至关重要的作用。作为吲哚生物碱最吸引人的亚型之一,高度取代的四氢-γ-咔啉是许多天然产物和药物中的基本结构单元。然而,由容易获得的试剂合成具有高官能度的四氢-γ-咔啉是巨大的挑战。尤其是,含有多立体中心的四氢-γ-咔啉的立体发散性合成仍然非常困难。在本文中,我们报告了在高效的级联过程中,通过协同Cu / Ir催化剂体系从醛亚胺酯和吲哚基烯丙基碳酸酯得到的具有立体异构控制显着高水平的四氢-γ-咔啉的便利且立体发散的组装体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号