当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of the human MLL1 core complex bound to the nucleosome.
Nature Communications ( IF 14.7 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41467-019-13550-2
Sang Ho Park 1 , Alex Ayoub 2 , Young-Tae Lee 2 , Jing Xu 2 , Hanseong Kim 1 , Wei Zheng 3 , Biao Zhang 3 , Liang Sha 2 , Sojin An 1 , Yang Zhang 1, 3 , Michael A Cianfrocco 1, 4 , Min Su 4 , Yali Dou 1, 2 , Uhn-Soo Cho 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41467-019-13550-2
Sang Ho Park 1 , Alex Ayoub 2 , Young-Tae Lee 2 , Jing Xu 2 , Hanseong Kim 1 , Wei Zheng 3 , Biao Zhang 3 , Liang Sha 2 , Sojin An 1 , Yang Zhang 1, 3 , Michael A Cianfrocco 1, 4 , Min Su 4 , Yali Dou 1, 2 , Uhn-Soo Cho 1
Affiliation
|
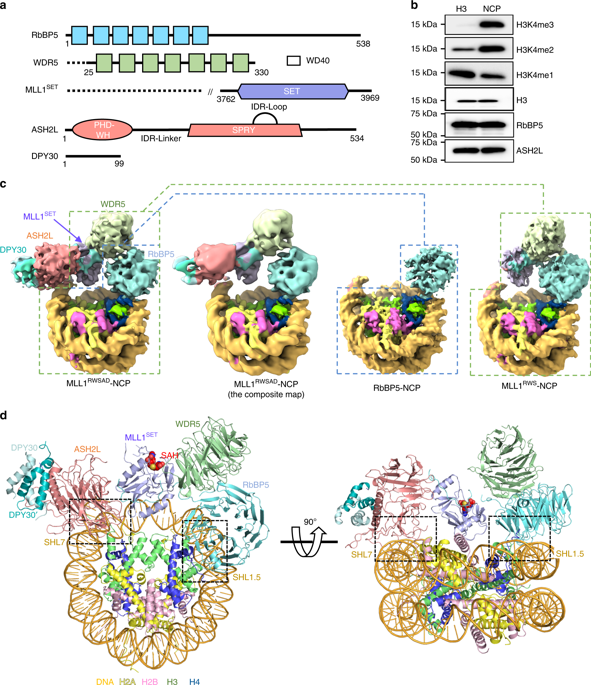
Mixed lineage leukemia (MLL) family histone methyltransferases are enzymes that deposit histone H3 Lys4 (K4) mono-/di-/tri-methylation and regulate gene expression in mammals. Despite extensive structural and biochemical studies, the molecular mechanisms whereby the MLL complexes recognize histone H3K4 within nucleosome core particles (NCPs) remain unclear. Here we report the single-particle cryo-electron microscopy (cryo-EM) structure of the NCP-bound human MLL1 core complex. We show that the MLL1 core complex anchors to the NCP via the conserved RbBP5 and ASH2L, which interact extensively with nucleosomal DNA and the surface close to the N-terminal tail of histone H4. Concurrent interactions of RbBP5 and ASH2L with the NCP uniquely align the catalytic MLL1SET domain at the nucleosome dyad, thereby facilitating symmetrical access to both H3K4 substrates within the NCP. Our study sheds light on how the MLL1 complex engages chromatin and how chromatin binding promotes MLL1 tri-methylation activity.
中文翻译:

与核小体结合的人 MLL1 核心复合物的冷冻电镜结构。
混合谱系白血病 (MLL) 家族组蛋白甲基转移酶是在哺乳动物中沉积组蛋白 H3 Lys4 (K4) 单甲基化/二甲基/三甲基化并调节基因表达的酶。尽管进行了广泛的结构和生化研究,但 MLL 复合物识别核小体核心颗粒 (NCP) 内的组蛋白 H3K4 的分子机制仍不清楚。在这里,我们报告了 NCP 结合的人类 MLL1 核心复合物的单粒子低温电子显微镜 (cryo-EM) 结构。我们表明 MLL1 核心复合物通过保守的 RbBP5 和 ASH2L 锚定到 NCP,它们与核小体 DNA 和靠近组蛋白 H4 N 末端尾部的表面广泛相互作用。RbBP5 和 ASH2L 与 NCP 的同时相互作用独特地排列了核小体 dyad 处的催化 MLL1SET 结构域,从而促进对 NCP 内两个 H3K4 底物的对称访问。我们的研究揭示了 MLL1 复合物如何与染色质结合,以及染色质结合如何促进 MLL1 三甲基化活性。
更新日期:2019-12-05
中文翻译:

与核小体结合的人 MLL1 核心复合物的冷冻电镜结构。
混合谱系白血病 (MLL) 家族组蛋白甲基转移酶是在哺乳动物中沉积组蛋白 H3 Lys4 (K4) 单甲基化/二甲基/三甲基化并调节基因表达的酶。尽管进行了广泛的结构和生化研究,但 MLL 复合物识别核小体核心颗粒 (NCP) 内的组蛋白 H3K4 的分子机制仍不清楚。在这里,我们报告了 NCP 结合的人类 MLL1 核心复合物的单粒子低温电子显微镜 (cryo-EM) 结构。我们表明 MLL1 核心复合物通过保守的 RbBP5 和 ASH2L 锚定到 NCP,它们与核小体 DNA 和靠近组蛋白 H4 N 末端尾部的表面广泛相互作用。RbBP5 和 ASH2L 与 NCP 的同时相互作用独特地排列了核小体 dyad 处的催化 MLL1SET 结构域,从而促进对 NCP 内两个 H3K4 底物的对称访问。我们的研究揭示了 MLL1 复合物如何与染色质结合,以及染色质结合如何促进 MLL1 三甲基化活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号