当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Investigation of a Hybrid Zn/V2O5 Rechargeable Battery with a Binary Li+/Zn2+ Aqueous Electrolyte
ChemSusChem ( IF 7.5 ) Pub Date : 2020-01-21 , DOI: 10.1002/cssc.201903072 Dauren Batyrbekuly 1, 2 , Sabrina Cajoly 1 , Barbara Laïk 1 , Jean‐Pierre Pereira‐Ramos 1 , Nicolas Emery 1 , Zhumabay Bakenov 2 , Rita Baddour‐Hadjean 1
ChemSusChem ( IF 7.5 ) Pub Date : 2020-01-21 , DOI: 10.1002/cssc.201903072 Dauren Batyrbekuly 1, 2 , Sabrina Cajoly 1 , Barbara Laïk 1 , Jean‐Pierre Pereira‐Ramos 1 , Nicolas Emery 1 , Zhumabay Bakenov 2 , Rita Baddour‐Hadjean 1
Affiliation
|
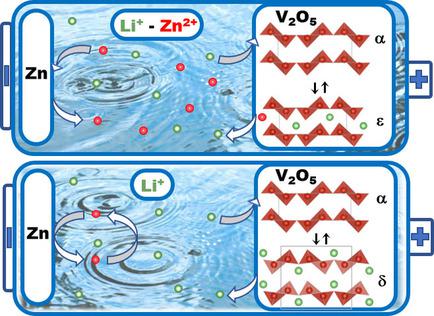
Low‐cost, easily processable, and environmentally friendly rechargeable aqueous zinc batteries have great potential for large‐scale energy storage, which justifies their receiving extensive attention in recent years. An original concept based on the use of a binary Li+/Zn2+ aqueous electrolyte is described herein for the case of the Zn/V2O5 system. In this hybrid, the positive side involves mainly the Li+ insertion/deinsertion reaction of V2O5, whereas the negative electrode operates according to zinc dissolution–deposition cycles. The Zn//3 mol L−1 Li2SO4–4 mol L−1 ZnSO4///V2O5 cell worked in the narrow voltage range of 1.6–0.8 V with capacities of approximately 136–125 mA h g−1 at rates of C/20–C/5, respectively. At 1 C, the capacity of 80 mA h g−1 was outstandingly stable for more than 300 cycles with a capacity retention of 100 %. A detailed structural study by XRD and Raman spectroscopy allowed the peculiar response of the V2O5 layered host lattice on discharge–charge and cycling to be unraveled. Strong similarities with the well‐known structural changes reported in nonaqueous lithiated electrolytes were highlighted, although the emergence of the usual distorted δ‐LiV2O5 phase was not detected on discharge to 0.8 V. The pristine host structure was restored and maintained during cycling with mitigated structural changes leading to high capacity retention. The present electrochemical and structural findings reveal a reaction mechanism mainly based on Li+ intercalation, but co‐intercalation of a few Zn2+ ions between the oxide layers cannot be completely dismissed. The presence of zinc cations between the oxide layers is thought to relieve the structural stress induced in V2O5 under operation, and this resulted in a limited volume expansion of 4 %. This fundamental investigation of a reaction mechanism operating in an environmentally friendly aqueous medium has not been reported before.
中文翻译:

具有二元Li + / Zn2 +水性电解质的Zn / V2O5混合充电电池的机理研究
低成本,易于加工且环保的可充电含水锌电池在大规模储能方面具有巨大潜力,这证明近年来受到广泛关注是有道理的。对于Zn / V 2 O 5系统,本文描述了基于使用二元Li + / Zn 2+水性电解质的原始概念。在这种混合体中,正极主要涉及V 2 O 5的Li +插入/插入反应,而负极根据锌的溶解-沉积循环运行。Zn // 3 mol L -1 Li 2 SO 4 –4 mol L -1ZnSO 4 / // V 2 O 5电池在1.6-0.8 V的窄电压范围内工作,在C / 20-C / 5的速率下,容量分别约为136-125 mA h g -1。在1 C时,80 mA h g -1的容量在300多个循环中非常稳定,容量保持率为100%。通过XRD和拉曼光谱的详细结构研究,可以阐明V 2 O 5层状主晶格在放电和循环过程中的特殊响应。在非水电解质锂化报告的公知的结构变化的相似性强的突出强调,虽然通常的扭曲δ-LIV出现2 ø 5放电至0.8 V时未检测到相。原始主体结构在循环过程中得以恢复和维持,结构变化减轻,从而导致高容量保留。目前的电化学和结构发现揭示了主要基于Li +嵌入的反应机理,但是不能完全消除氧化物层之间少量Zn 2+离子的共嵌入。认为在氧化物层之间存在锌阳离子可减轻在操作中在V 2 O 5中引起的结构应力,并且这导致4%的有限体积膨胀。以前尚未报道过在环境友好的水性介质中进行反应的机理的基础研究。
更新日期:2020-01-22
中文翻译:

具有二元Li + / Zn2 +水性电解质的Zn / V2O5混合充电电池的机理研究
低成本,易于加工且环保的可充电含水锌电池在大规模储能方面具有巨大潜力,这证明近年来受到广泛关注是有道理的。对于Zn / V 2 O 5系统,本文描述了基于使用二元Li + / Zn 2+水性电解质的原始概念。在这种混合体中,正极主要涉及V 2 O 5的Li +插入/插入反应,而负极根据锌的溶解-沉积循环运行。Zn // 3 mol L -1 Li 2 SO 4 –4 mol L -1ZnSO 4 / // V 2 O 5电池在1.6-0.8 V的窄电压范围内工作,在C / 20-C / 5的速率下,容量分别约为136-125 mA h g -1。在1 C时,80 mA h g -1的容量在300多个循环中非常稳定,容量保持率为100%。通过XRD和拉曼光谱的详细结构研究,可以阐明V 2 O 5层状主晶格在放电和循环过程中的特殊响应。在非水电解质锂化报告的公知的结构变化的相似性强的突出强调,虽然通常的扭曲δ-LIV出现2 ø 5放电至0.8 V时未检测到相。原始主体结构在循环过程中得以恢复和维持,结构变化减轻,从而导致高容量保留。目前的电化学和结构发现揭示了主要基于Li +嵌入的反应机理,但是不能完全消除氧化物层之间少量Zn 2+离子的共嵌入。认为在氧化物层之间存在锌阳离子可减轻在操作中在V 2 O 5中引起的结构应力,并且这导致4%的有限体积膨胀。以前尚未报道过在环境友好的水性介质中进行反应的机理的基础研究。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号